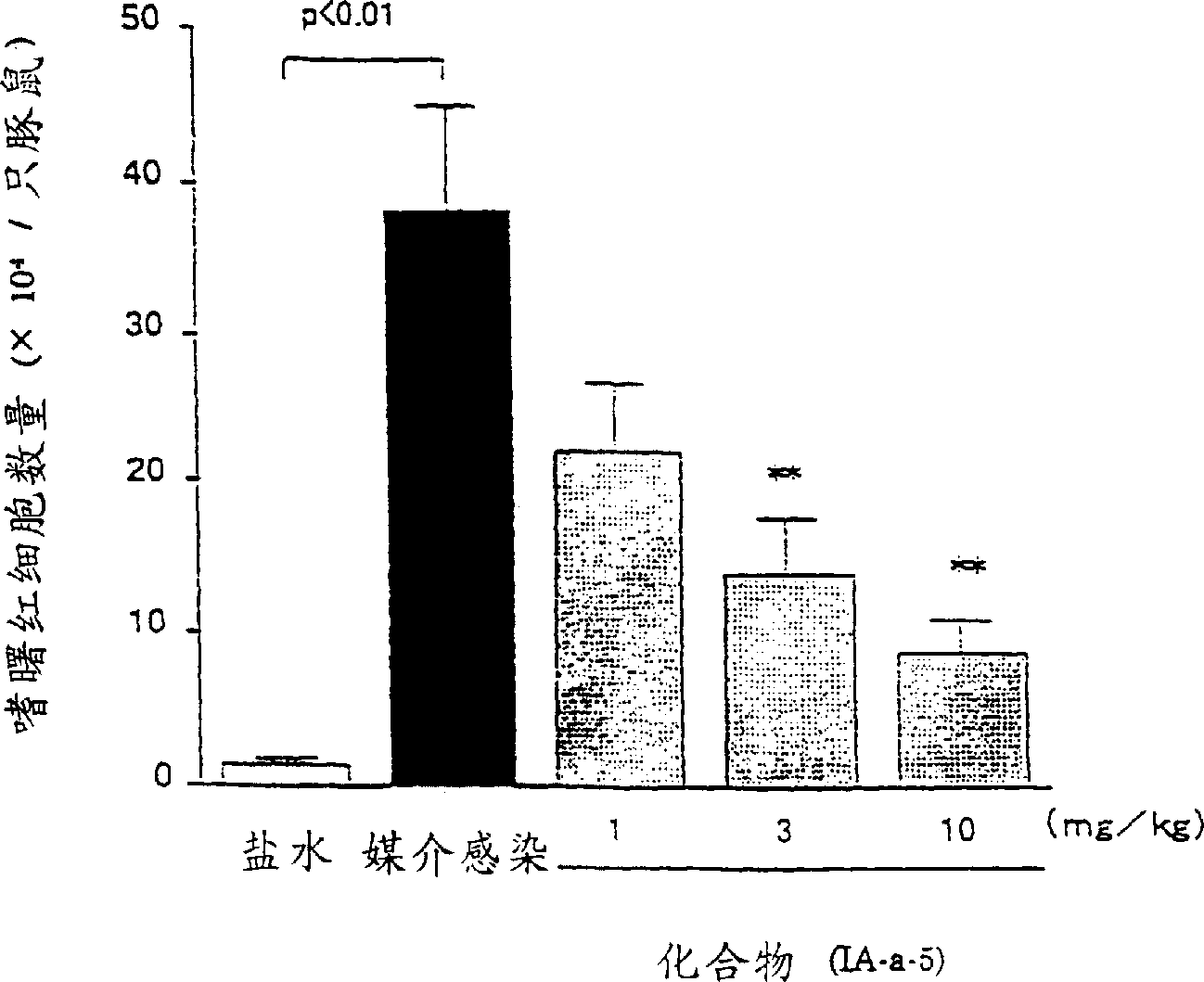
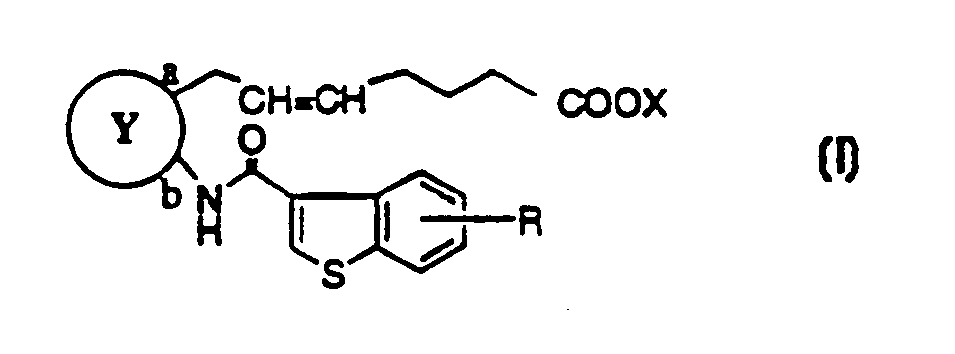
Benzothiophenecarboxamide derivatives and PGD2 antagonists comprising them
A technology of antagonists and compounds, which is applied in the field of benzothiophene amide derivatives and their intermediates, can solve the problems such as the great influence on prostaglandin synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0069] Preparation of (1R, 2S, 3S, 5S)-2-(2-amino-6,6-dimethylbicyclo[3.1.1]hept-3-yl)ethanol (IVA-b-1) and (IR, 2R , 3S, 5S)-2-(2-amino-6,6-dimethylbicyclo[3.1.1]hept-3-yl)ethanol (IVA-c-1)
[0070] According to the literature method, compound (6) (Chem.Pharm.Bull.Vol.37, No. 6 1524-1533 (1989)) can be reduced with sodium, and compound (IVA-a-1) can be removed by filtration as benzoate. The mother liquor (79 g) was suspended in 150 ml of ethyl acetate, 260 ml of 1N HCl was added and stirred. The aqueous phase separated from the two phases was basified with 65 ml of 4N NaOH solution and extracted with ethyl acetate. The organic phase was washed with water, dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The resulting oily residue (6.7 g out of 30 g) was dissolved in 40 ml of 90% methanol and washed with 500 ml of ion exchange resin-Amberlite CG-50 (NH 4 + ) type I absorption and was eluted with a gradient of 2.2 liters of water and 2.2 lit...
Embodiment 1
[0074] Preparation of (5Z)-7-{(1R,2R,3S,5S)-2-(5-Hydroxybenzo[b]thiophen-3-ylcarbonylamino)-6,6-dimethylbicyclo[3.1.1 Sodium ]hept-3-yl}-5-heptenoate (IVA-a-6) (step 1)
[0075] To a solution of 1.450 mg (5.2 mmol) of compound (IIA-a-1) (see Japanese Patent Publication (Kokoku) No. 23170 / 1994) in 25 ml of tetrahydrofuran, 2.6 ml (18.7 mmol) of triethylamine and 1.454 mg (1.1 mmol) of ) 5-acetoxybenzo[b]thiophene-3-carbonyl chloride (5) (prepared by Reference Example 2) was stirred for 1.5 hours, and the mixture was diluted with water and extracted with toluene. The organic phase was washed with dilute hydrochloric acid and water, dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was chromatographed on silica gel (eluent toluene:ethyl acetate=9:1) to obtain 2.481 mg of compound IA-a-10). Yield 96.1%. [α] D 23 =+48.0° (c=1.01%, CH 3 OH) elemental analysis (C 28 h 35 NO 5 S·0.1H 2 O) Theoretical (%): C, 67.34; H, 7.10; N, 2.80...
Embodiment 2
[0078] Preparation of (5Z)-7-[(1R,2S,3R,5S)-2-(5-Hydroxybenzo[b]thiophen-3-yl-carbonylamino)-6,6-dimethyl-bicyclo[3.1 .1]hept-3-yl]-5-heptenoic acid (IA-b-1) (step 1)
[0079] To 916mg (3mmol) (1R, 2S, 3S, 5S)-2-(2-amino-6,6-dimethylbicyclo[3.1.1]hept-3-yl)ethanol benzoate in 3ml of water 3.1 ml of 1N HCl was added to the suspension. The precipitated benzoic acid was extracted with ethyl acetate. The aqueous phase was adjusted to pH 10.5 with 700 mg of anhydrous sodium carbonate, and a solution of 1.06 g (3 mmol) of 5-benzenesulfonyloxybenzo[b]thiophene-3-carbonyl chloride (3) in 6 ml of tetrahydrofuran was added dropwise thereto. After 1.5 hours, the mixture was diluted with water and extracted with toluene. The organic phase was washed with water, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The obtained residue (1.5 g) was subjected to silica gel chromatography (eluent: hexane: ethyl acetate = 1:1) to obtain 1.497 g of compound (VA-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com