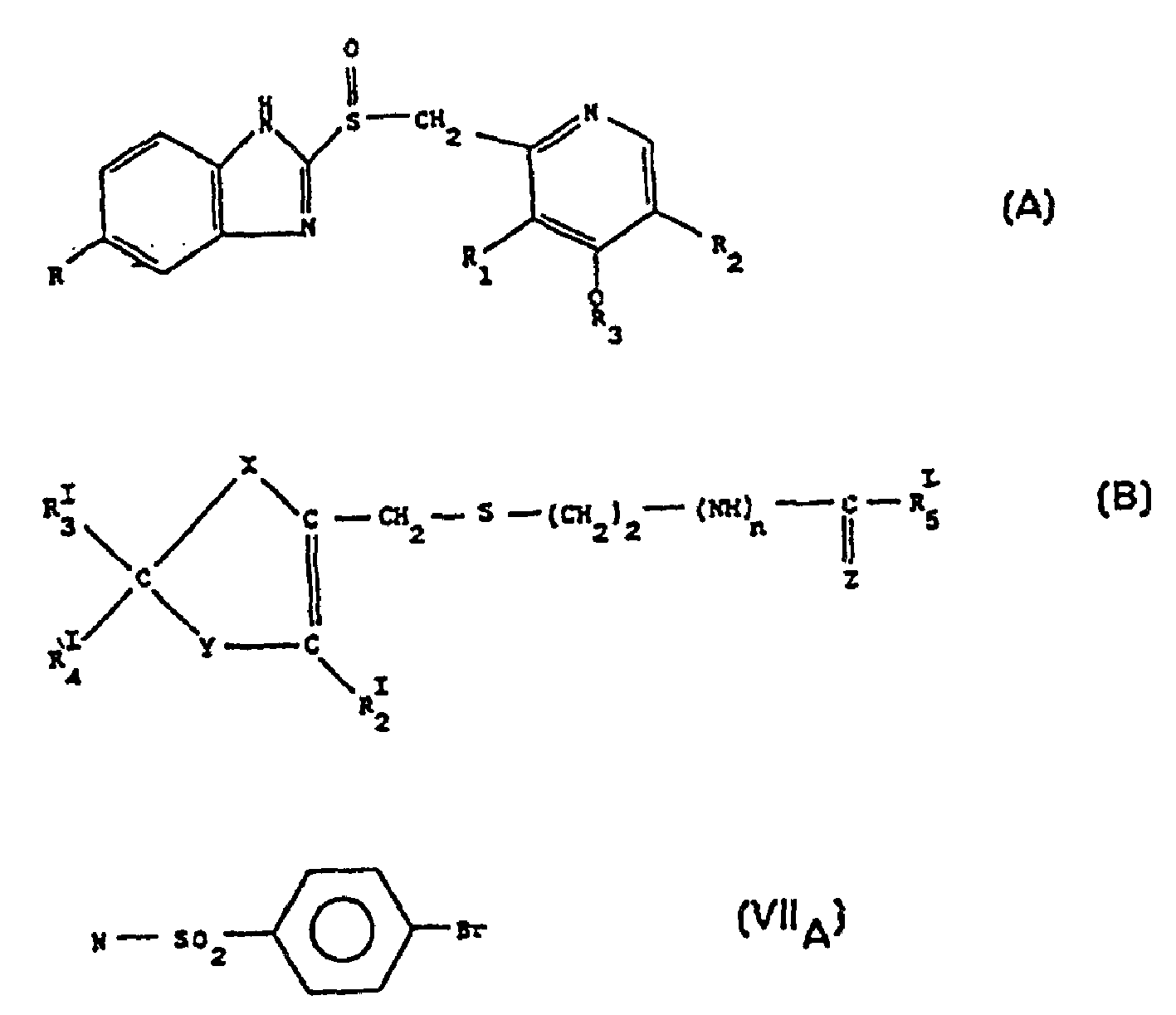
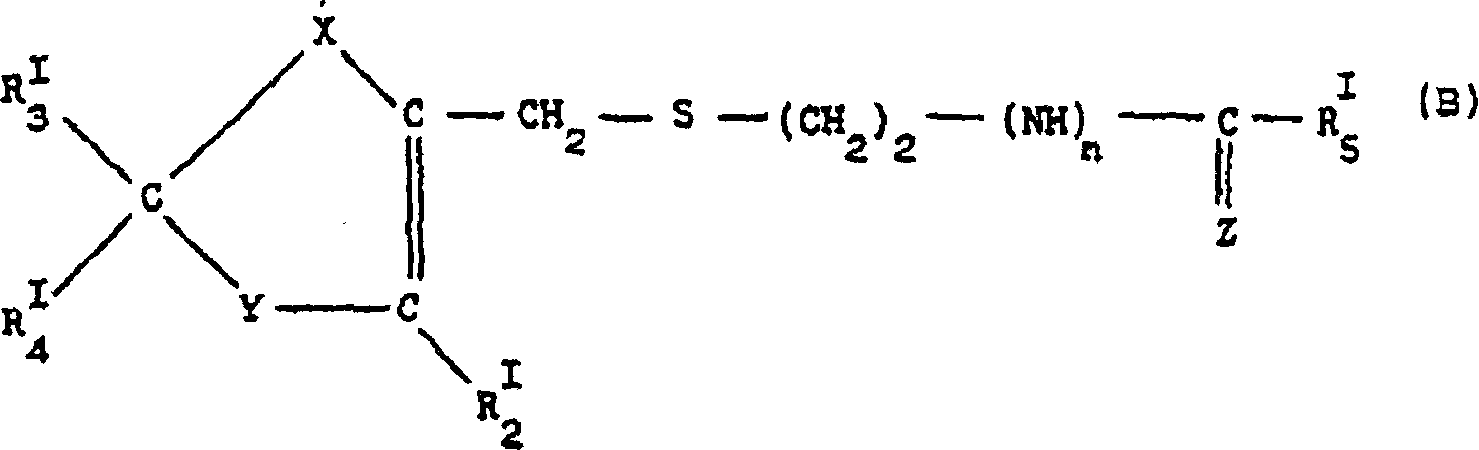Nitrate salt of anti-ulcer medicine
A technology of nitrates and drugs, used in the treatment and prevention of ulcer recurrence and most dyspepsia compositions, gastroprotective activity and anti-high gastric acid secretion active composition field, can solve the problem of unsatisfactory tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of cimetidine nitrate
[0051] 10 g of cimetidine was dissolved in 100 ml of acetonitrile / tetrahydrofuran / water 1:1:2 (volume ratio) mixture cooled at 4°C. A solution of 2.5 mL of 70% nitric acid in 10 mL of acetonitrile was gradually added. The solution was diluted with ether and the temperature was maintained at 4°C until the product started to precipitate. After several hours the precipitated solid was filtered off, washed with ether and dried. 12.1 g of cimetidine mononitrate were recovered, with a melting point of 158-159° C. (decomposition).
[0052] 1 H-NMR (D 2 O): 8,55 (1H, s), 3,83 (2H, s), 3,32 (2H, s), 2,77 (3H, s), 2,68 (2H, t), 2, 32(3H,s).
[0053] Elemental analysis:
[0054] Calculated value (%) C 38.09 H 5.43 N 31.09 S 10.17
[0055] Found value (%) C 37.99 H 5.41 N 31.16 S 10.25
Embodiment 2
[0057] Preparation of ranitidine nitrate
[0058] 5 g of ranitidine hydrochloride were dissolved in 140 ml of acetonitrile / methanol 6:1 mixture at 20°C. Add 4.2 grams of silver nitrate powder. The precipitated silver chloride is filtered off, washed with an acetonitrile / methanol 6:1 solution, the organic phases are combined, dried and worked up to give a dry residue. An amorphous solid corresponding to ranitidine mononitrate was thus obtained.
[0059] 1 H-NMR (D 2 O): 6,70(1H,d), 6,40(1H,d), 4,34(2H,s), 3,83(2H,s), 3,43(2H,t), 2, 93(2H, m), 2,87(9H, s).
[0060] Calculated value (%) C 41.37 H 6.14 N 18.56 S 8.50
[0061] Measured value (%) C 41.12 H 6.20 N 18.44 S 8.38
[0062] Pharmacological experiment
Embodiment 3
[0064] acute toxicity
[0065] In the form of a 2% w / v carboxymethylcellulose aqueous suspension, a group of 10 rats each weighing 20 g were orally administered a single dose of 100 mg / kg of the western medicine prepared in the preceding examples via intubation. Metidine and ranitidine nitrate.
[0066] These experimental animals were monitored for 14 days. No signs of toxicity were observed in any of the animals in this group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com