Vaginal lactobacillus medicant
A technology of Lactobacillus and medicine, applied in the field of prevention and treatment of vaginal infection, can solve the problems of infection, prevention or treatment of vaginal infection with little effect, lack of ability of microorganisms to combine and settle, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
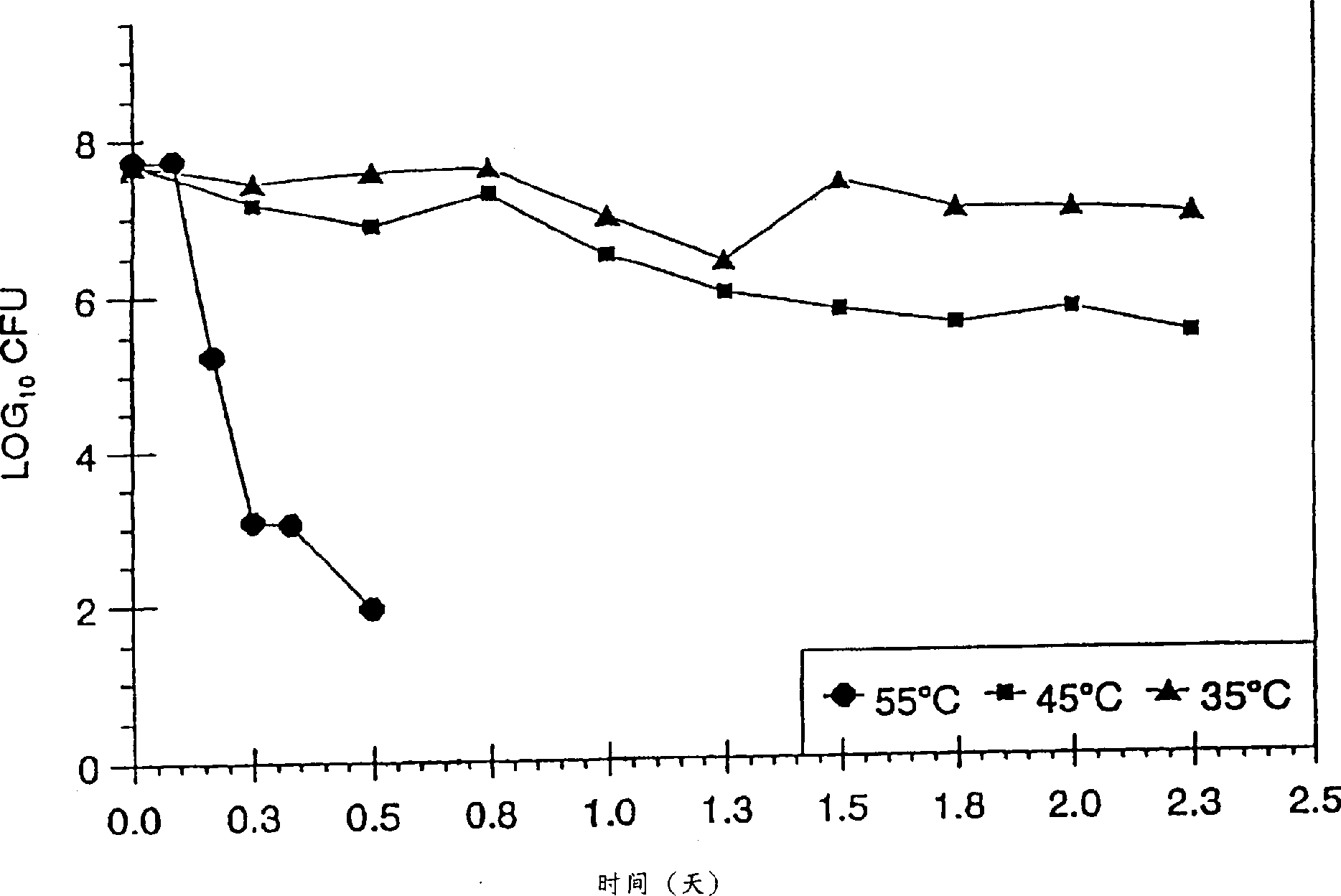
[0114] The following examples demonstrate methods for selecting Lactobacillus strains suitable for use in the present invention.
[0115] Six Lactobacillus strains were isolated from the vagina of six healthy, asexual women, and a series of beneficial properties of these six different Lactobacillus strains were evaluated. The purity of these 6 strains, the ability to adhere to vaginal epithelial cells, the stability of genetic properties, the ability to produce hydrogen peroxide, and the survival stability of long-term storage need to be evaluated. Suitable strains for inclusion should possess: purity (i.e., freedom from contaminating microorganisms), as determined by the ability to maintain substantially pure cultures of the parental strain; a percent vaginal epithelial cell adhesion value of at least approximately 80%; stable genetic performance , by identifying strains in which the genetic properties of the cultured cells are the same as the parental strain, thereby assessi...
Embodiment 2
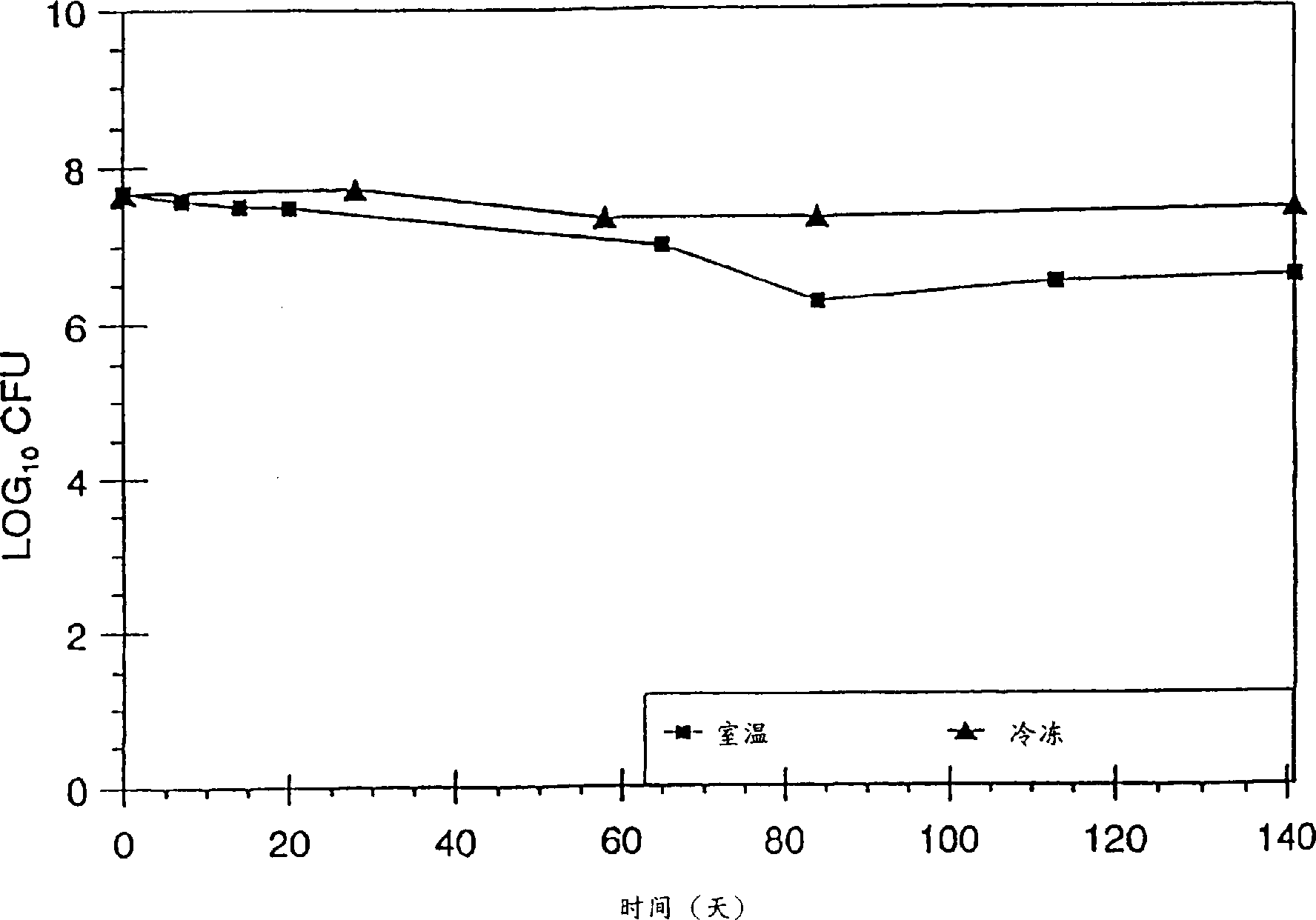
[0133] The following examples represent the determination of the optimum ratio of cells to preservation matrix that provides high viable numbers of cells of the preserved strain CTV-05.
[0134] plate number
Embodiment 3
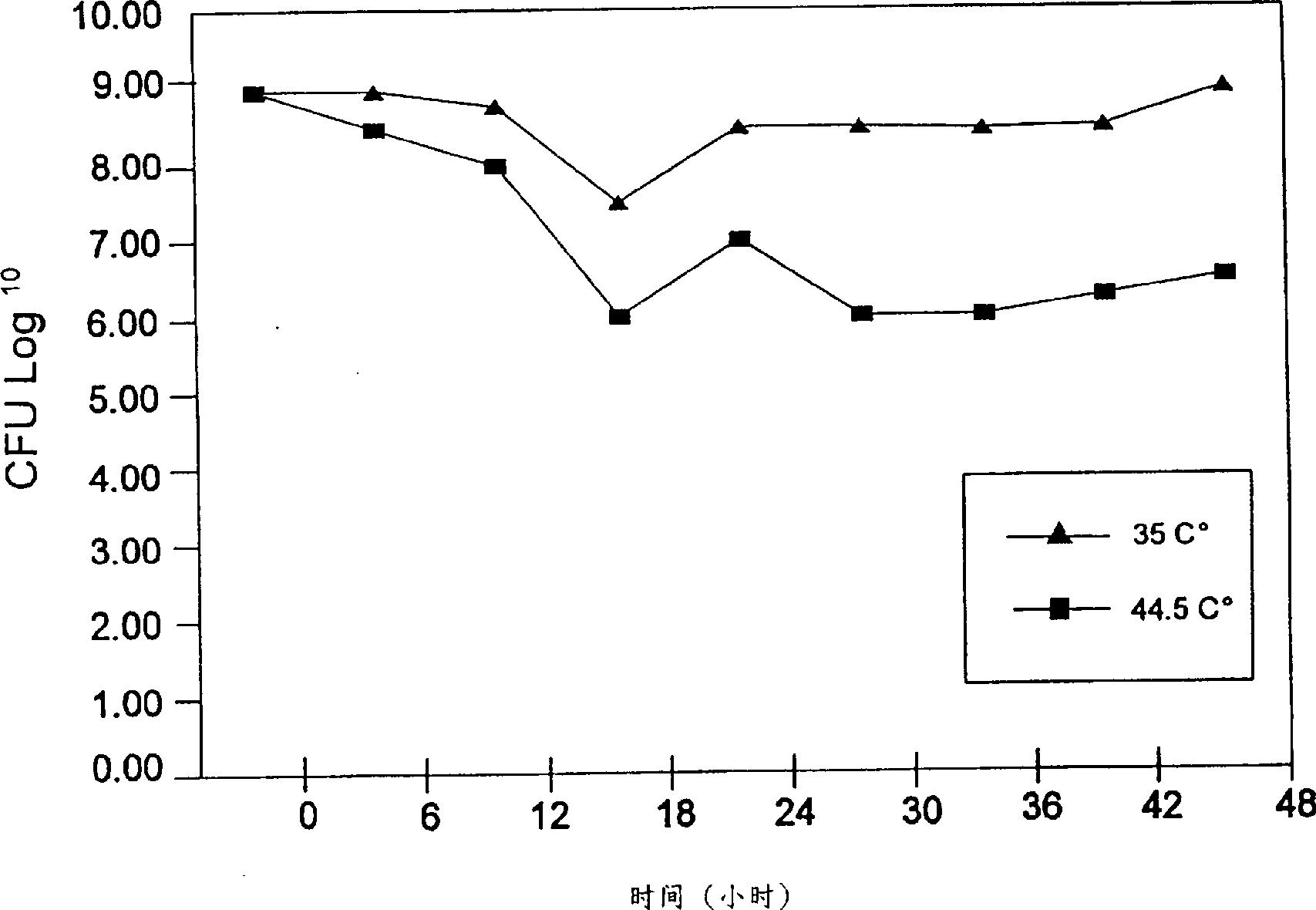
[0136] This example describes a specific method for preparing the vaginal medicine of the present invention.
[0137] The composition of the MRS medium used in all experiments was as follows: 10 g hydrazone peptone No. 3 for bacteria, 10 g beef extract for bacteria, 5 g yeast extract for bacteria, 20 g glucose extract for bacteria, 1 g Tween 80, 2 g ammonium citrate , 5g sodium acetate, 0.1g magnesium sulfate, 0.05g manganese sulfate and 2g dipotassium hydrogen phosphate, dilute the above components to 1000ml with reagent grade water. The so-called MRS agar refers to adding 10 g of agar to the above mixture. The medium was adjusted to pH 6.5±0.2 at 25°C.
[0138] Preservation matrices were prepared as follows. 2X gelatin (eg 137.5g / 500ml reagent grade water) and 4X skim milk (eg 15g / 250ml reagent grade water) were autoclaved at about 121°C for about 15 minutes. Mix together 4X xylitol (eg 59g / 250ml reagent grade water) and 4X glucose (eg 25g / 250ml reagent grade water), adju...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com