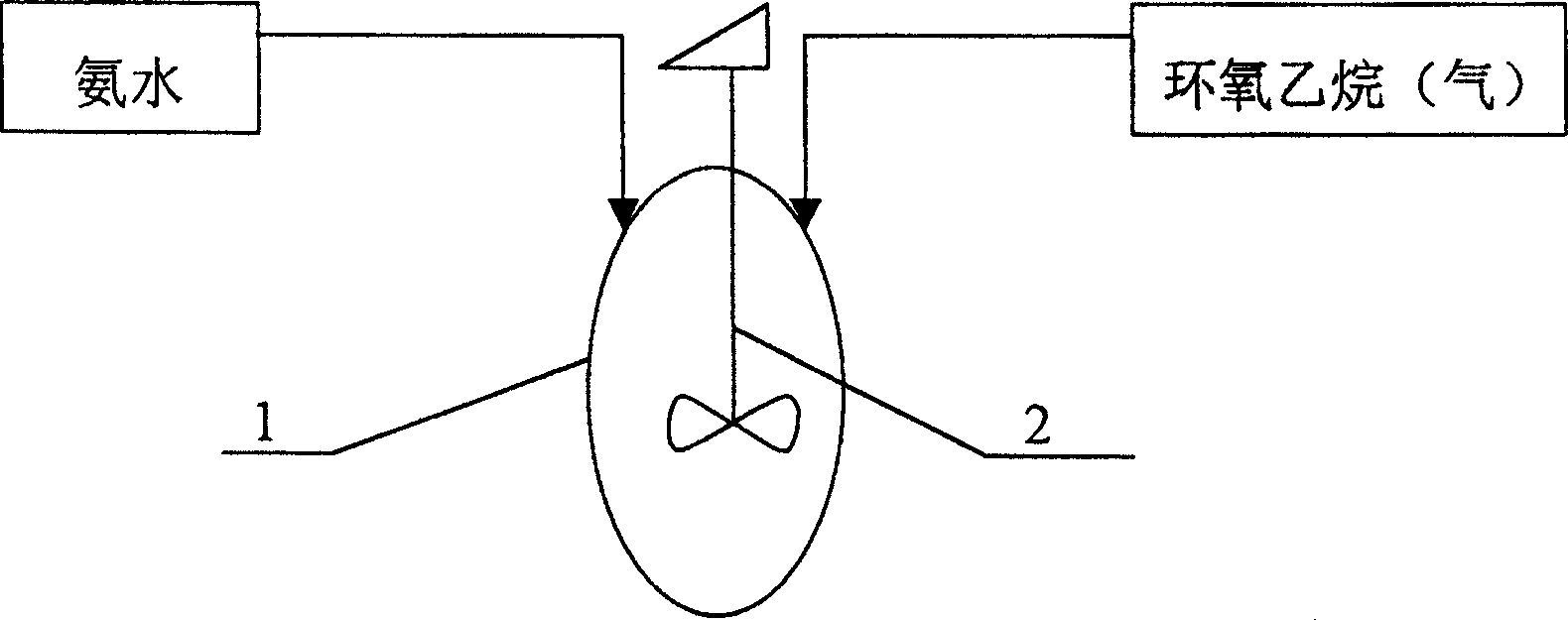
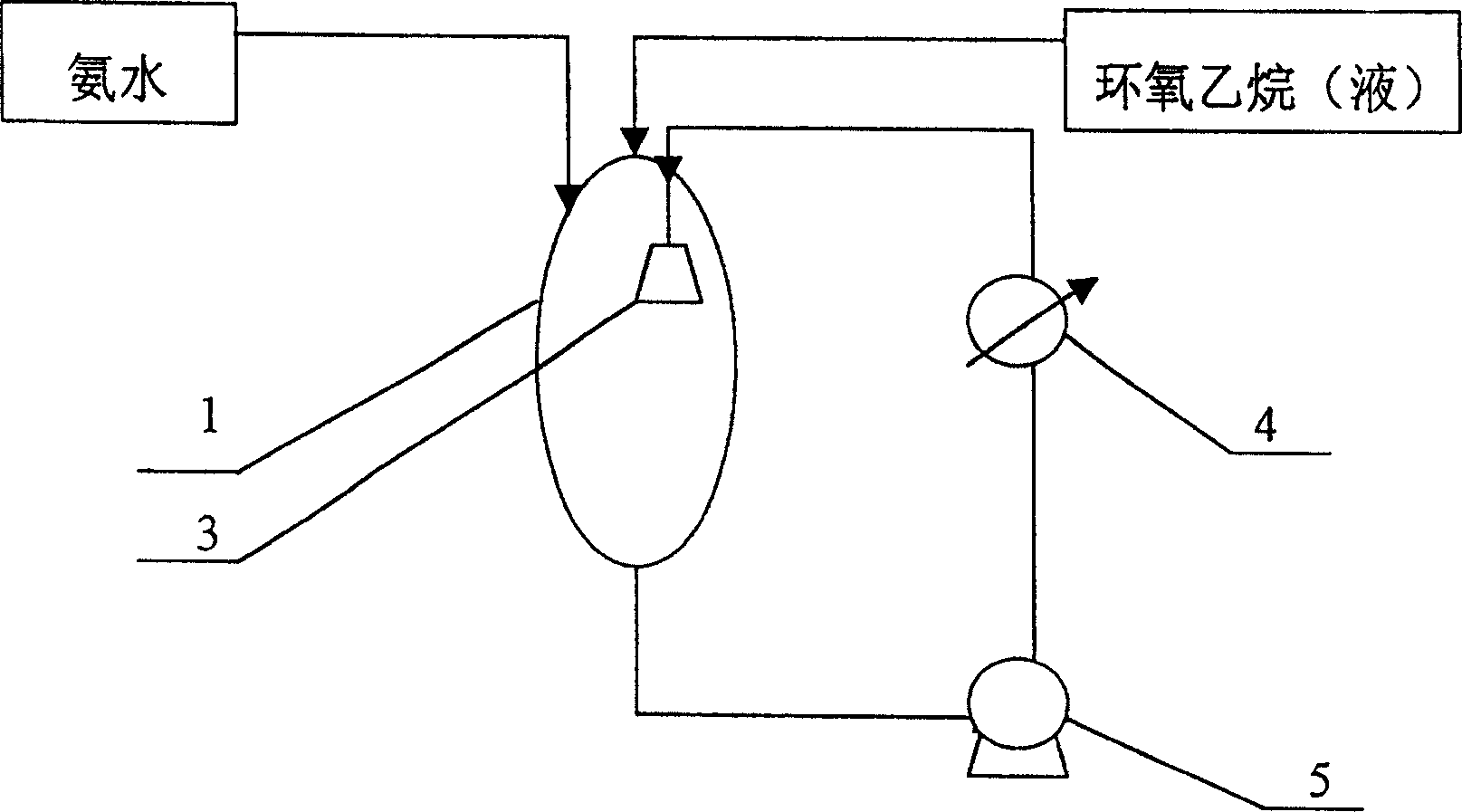
Production of triethanolamine products
A technology of triethanolamine and triethanolamine stock solution, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as increased negative reactions, high reaction temperature, easy backflow of materials, etc., and achieve rapid and complete reactions, increased Effects of large specific surface area and improved product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment one: 250Kg liquefied ammonia is mixed with concentration and is the ammoniacal solution of 25%, injects in the reaction vessel, then directly pressurizes 1400Kg ethylene oxide and injects in the reaction vessel and reacts with the ammoniacal solution by metering tank again; The pump 5 removes the material in the reaction vessel 1, cools down in the heat exchanger 4, and then sprays it into the reaction vessel through the static atomization injector 3 installed in the reaction vessel. This cycle accelerates the reaction of the material, and the reaction is complete, and triethanolamine is produced. Stock solution; the entire reaction time is 2.5 hours, the temperature in the reaction vessel 1 is 60°C, and the reaction pressure is 0.1Mpa. The obtained triethanolamine stock solution was distilled in a distillation pot for about 2 hours to obtain 1630 Kg of a triethanolamine product with an effective content of triethanolamine of 85%.
Embodiment 2
[0024] Embodiment two: 250Kg liquefied ammonia is mixed with concentration and is the ammoniacal solution of 25%, injects in the reaction vessel, then directly pressurizes 1425Kg ethylene oxide and injects in the reaction vessel and reacts with the ammoniacal solution by metering tank again; The pump 5 removes the material in the reaction vessel 1 and sprays it into the reaction vessel 1 through the static atomization injector 3 provided in the reaction vessel after being cooled by the heat exchanger 4. This circulation makes the reaction of the material accelerate and the reaction is complete, and three Ethanolamine stock solution; the entire reaction time is 3 hours, the temperature in the reaction vessel is 60°C, and the reaction pressure is 0.06Mpa. The obtained triethanolamine stock solution was distilled in a distillation pot for about 2 hours to obtain 1660 Kg of a triethanolamine product with an effective content of triethanol of 85.2%.
Embodiment 3
[0025] Embodiment three: 250Kg liquefied ammonia is mixed with concentration and is the ammoniacal solution of 25%, injects in the reaction vessel, then directly pressurizes 1450Kg ethylene oxide and injects in the reaction vessel and reacts with the ammoniacal solution by metering tank again; Then by external circulation The pump 5 removes the material in the reaction vessel 1 and sprays it into the reaction vessel through the static atomization injector 3 provided in the reaction vessel after being cooled by the heat exchanger 4. This circulation makes the reaction speed of the material faster and the reaction is complete, and three Ethanolamine stock solution; the entire reaction time is 2 hours, the temperature in the reaction vessel is 60°C, and the reaction pressure is 0.2Mpa. The obtained triethanolamine stock solution was distilled in a distillation pot for about 2 hours to obtain 1670 Kg of a triethanolamine product with an effective content of triethanolamine of 85.3%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com