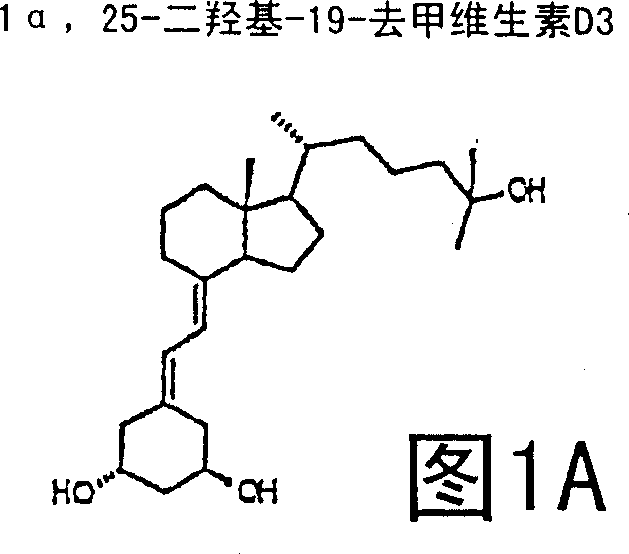
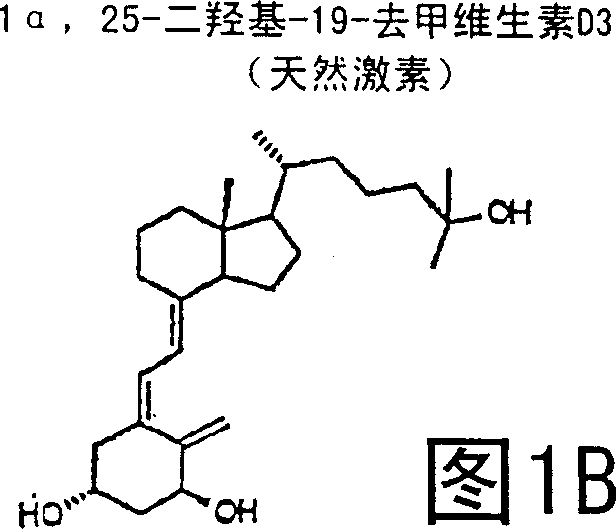
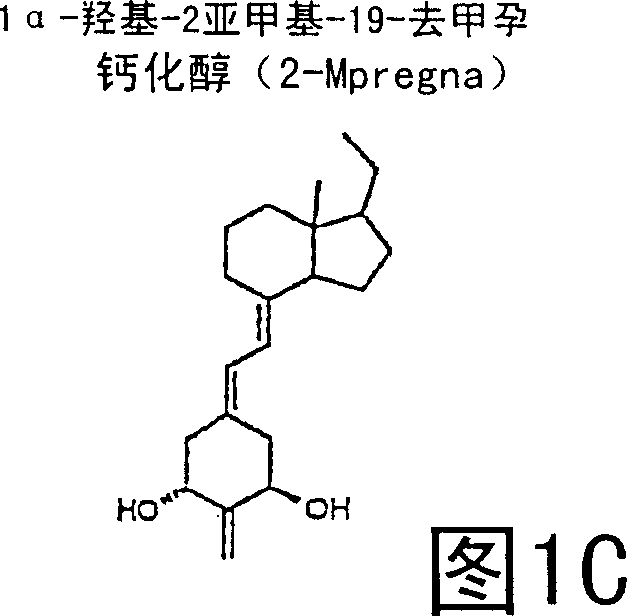
(20s)-1alpha-hydroxy-2-methylene-19-nor-bishomopregnacalciferol and its uses
A methylene, calciferol technology, applied in organic chemistry, digestive system, medical preparations containing active ingredients, etc., can solve problems such as the inability to meet the purity standards of therapeutic drugs, achieve low cost, increase moisture, and improve barrier function Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] recrystallized from acetone
[0089] (a) Dissolve the desired purified 2-MbisP product in boiling acetone (1.2ml, Aldrich) under an argon atmosphere, place it at room temperature (68°F) for several hours (1-3 hours), and then place it in the refrigerator (35~45) overnight (8~12 hours). The precipitated crystals were filtered, washed with a small amount of cold (0°C) acetone, and dried.
[0090] (b) The 2-MbisP crystals were then recrystallized from acetone (0.5 ml) as described in step 1(a). The precipitated crystals have a relatively accurate melting point (relatively narrow melting range): 162-164°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com