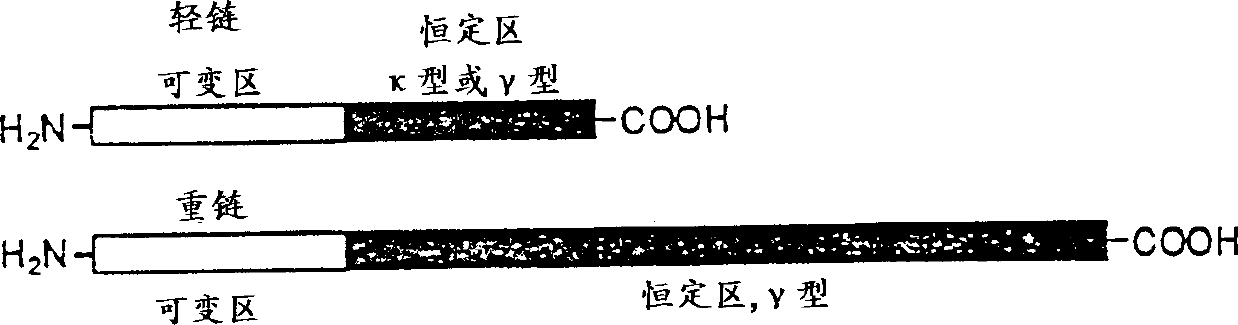
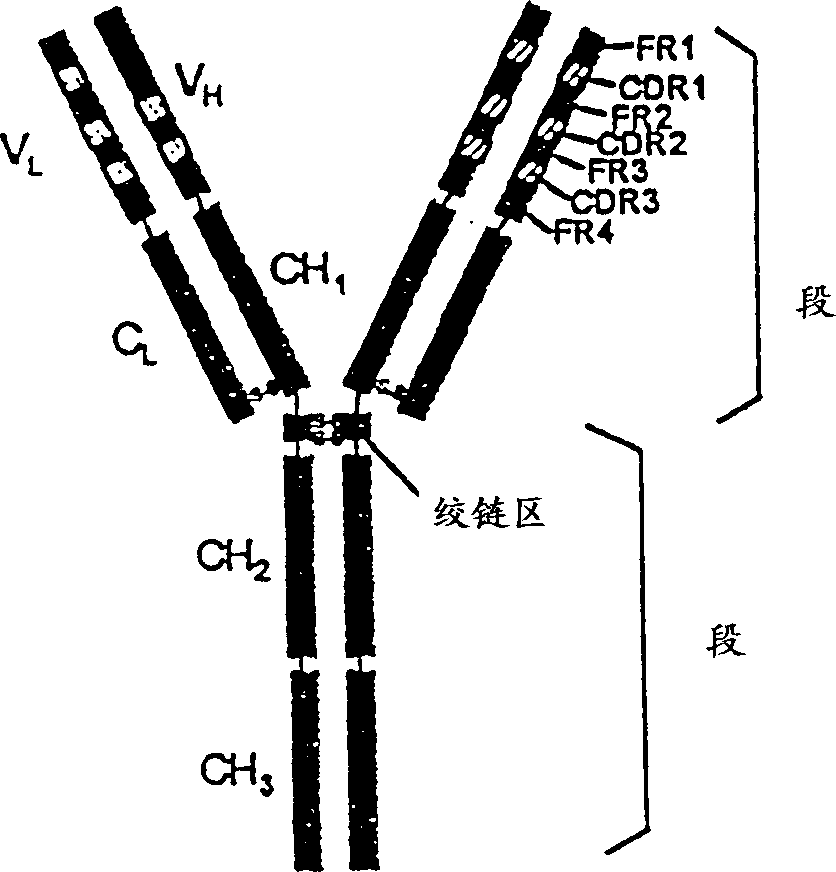
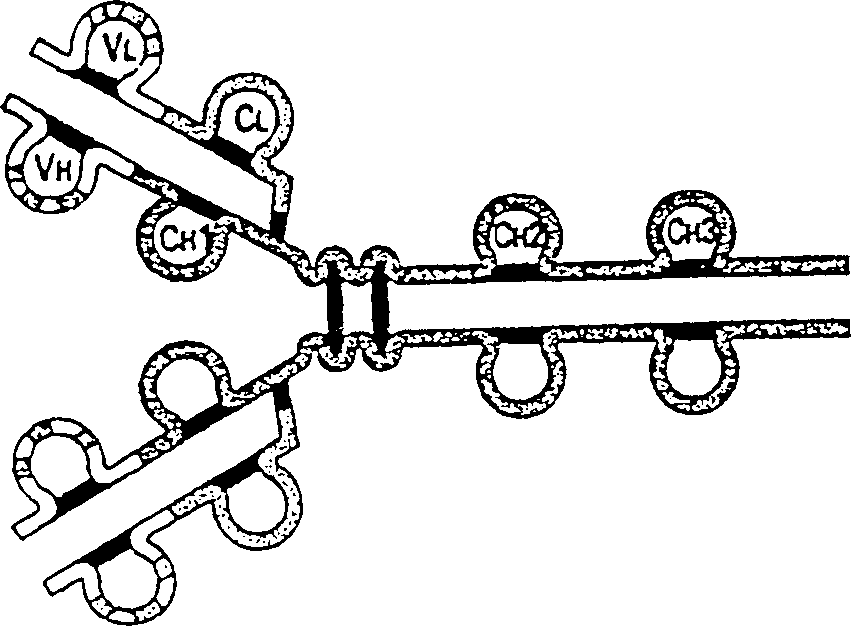
Modified antibodies of induced anti-idiotype reaction enhancement
An anti-idiotype and reaction technology, applied in the field of modified immunoglobulin and its vaccine composition, can solve the problem of limited tendency of anti-idiotype reaction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0116] Alternatively, disulfide-forming cysteines may be replaced with non-sulfhydryl-containing atypical amino acids or chemical amino acid analogs (such as, but not limited to, conventional protein synthesis methods). Atypical amino acids include: D-amino acids of typical amino acids, α-aminobutyric acid, 4-aminobutyric acid, Abu, 2-aminobutyric acid, γ-Abu, ε-Ahx, -aminocaproic acid, Aib, 2-aminoisobutyric acid Butyric acid, 3-aminopropionic acid, ornithine, norleucine, norvaline, hydroxyproline, sarcosine, citrulline, tert-butylglycine, tert-butylalanine, phenyl Glycine, cyclohexylalanine, β-alanine, fluoro-amino acids such as designed β-methyl amino acids, Cα-methyl amino acids, Nα-methyl amino acids, amino acid analogs. Also, amino acids can be right-handed or left-handed. In alternative embodiments, disulfide bond forming amino acid residues may be deleted.
[0117] In specific embodiments, disulfide bond-forming amino acid residues in the variable region of the heavy...
Embodiment 7
[0364] Example 7 Expression of Synthetic Modified Antibody Containing HMFG-1 Sequence
[0365]Anti-idiotypic antibodies that immunospecifically bind to HMFG-1 antibodies were constructed. HMFG-1 is an antibody known to bind polymorphic epidermal mucin (PEM) (Stewart et al., 1990, Journal of Clinical Oncology 8: 1941-50; Kosmas et al., 1994, Cancer 73: 3000-3010 ). The antigenic determinant sequence ProAspThrArgPro of PEM is inserted into the variable region of the antibody by the method of the present invention. This short sequence is a highly immunogenic region in human polymorphic epidermal mucin (Gendler et al., 198, J. Biol. Chem. 163: 12820-12823). The 27A-27E (SerLeuLeuTyrSer) residues of HMFG-1 (Table 6) were replaced with ProAspThrArgPro by the oligonucleotide synthesis method described in Section 6, see FIG. 10 . Using the known gene sequence of the light and heavy chain variable regions of the HMFG-1 antibody, an anti-idiotypic antibody that immunospecifically bin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com