Peptide derivative
A peptide chain and formula technology, applied in the field of new peptides, can solve problems such as unknown ligands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
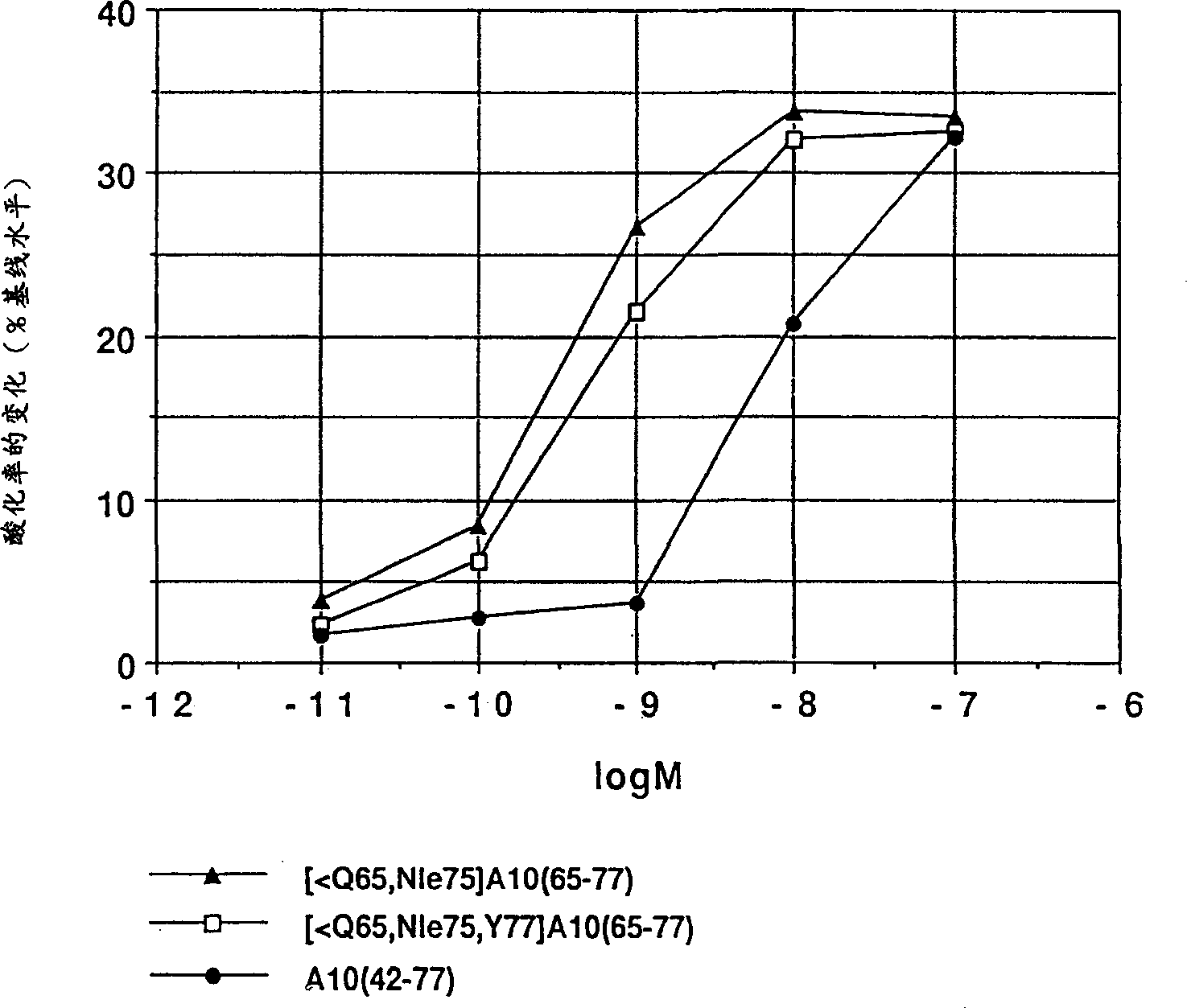
Image
Examples
Embodiment 1
[0398] Example 1 (Leu-Val-Gln-Pro-Arg-Gly-Ser-Arg-Asn-Gly-Pro-Gly-Pro-Trp-Gln-Gly-Gly-Arg-Arg-Lys-Phe-Arg-Arg- Production of Gln-Arg-Pro-Arg-Leu-Ser-His-Lys-Gly-Pro-Nle-Pro-Phe)
[0399] Introduce Fmoc-Phe-OH into commercially available 2-chlorotrityl resin (Clt resin, 1.3mmol / g) to obtain 0.25mmol Fmoc-Phe-O-Clt resin (0.32mmol / g), take a portion into the peptide In the reaction box of the synthesizer ABI433A, solid-phase synthesis was performed by the Fmoc / DCC / HOBt method. As the protecting group for the side chain of Fmoc amino acid, Pbf group is used to protect Arg, tBu group is used to protect Ser, Boc group is used to protect Trp and Lys, and Trt group is used to protect His, Asn and Gln. The other amino acids used and unprotected side chains were introduced along the N-terminal direction (ie, the direction from Phe to Leu in the above sequence) to obtain the desired protected peptide resin.
[0400] After stirring 50mg (4.45mmol) of the above resin in a 1ml mixture of...
Embodiment 2
[0407] Example 2 (Leu-Val-Gln-Pro-Arg-Gly-Ser-Arg-Asn-Gly-Pro-Gly-Pro-Trp-Gln-Gly-Gly-Arg-Arg-Lys-Phe-Arg-Arg- Production of Gln-Arg-Pro-Arg-Leu-Ser-His-Lys-Gly-Pro-Nle-Pro-Tyr)
[0408] In Example 1, the amino acid to be introduced into 2-chlorotrityl resin (Clt resin, 1.3mmol / g) was replaced by Fmoc-Tyr(tBu)-OH, and the synthesis and purification were carried out in the same way, and 13.3 was obtained by lyophilization. mg of desired product as a white powder.
[0409] (M+H) + Analysis by mass spectrometry: 4192.0 (calculated value: 4192.3)
[0410] HPLC elution time: 16.9 minutes
[0411] Elution condition:
[0412] Column: YMC A-301-3 (4.6×100mm)
[0413] Eluent: with solution A: 0.1% TFA-water and solution B: acetonitrile containing 0.1% TFA, eluted with a linear concentration gradient of A / B: 100 / 0 to 50 / 50 (25 minutes)
[0414] Flow rate: 1.0ml / min.
Embodiment 3
[0415] Example 3 (production of pGlu-Arg-Pro-Arg-Leu-Ser-His-Lys-Gly-Pro-Nle-Pro-Phe)
[0416]0.25mmol of Fmoc-Gly-O-Clt resin (0.392mmol / g) obtained by introducing Fmoc-Phe-OH into commercially available 2-chlorotrityl resin (Clt resin, 1.3mmol / g) into a portion of peptide In the reaction box of synthesizer ABI433A, Fmoc-Lys(Boc), Fmoc-His(Trt), Fmoc-Ser(tBu), Fmoc-Leu, Fmoc-Arg(Pbf), Fmoc-Leu, Fmoc-Arg(Pbf), Fmoc-Pro, Fmoc-Arg(Pbf) and Boc-Gln to obtain the desired protected peptide resin.
[0417] After 1 g of resin was placed in 20 ml of a mixture of AcOH:TFA:DCM (1:2:7) and stirred at room temperature for two hours, the mixture was filtered to remove the resin, the solvent was evaporated and lyophilized to obtain 362 mg of the protected peptide (Boc-Gln-Arg(Pbf)-Pro-Arg(Pbf)-Leu-Ser(tBu)-His(Trt)-Lys(Boc)-Gly-OH).
[0418] H-Phe-Obzl-HCl was sequentially condensed with Boc-Pro, Boc-Nle and Boc-Pro to obtain 180 mg of Boc-Pro-Nle-Pro-Phe-Bzl.
[0419] 50 mg Boc-Gln-Arg(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com