Process for preparing tricyclic compound having antihistaminic activity
A kind of compound, cycloalkyl technology, applied in the field of improvement of preparing antihistamine drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example A
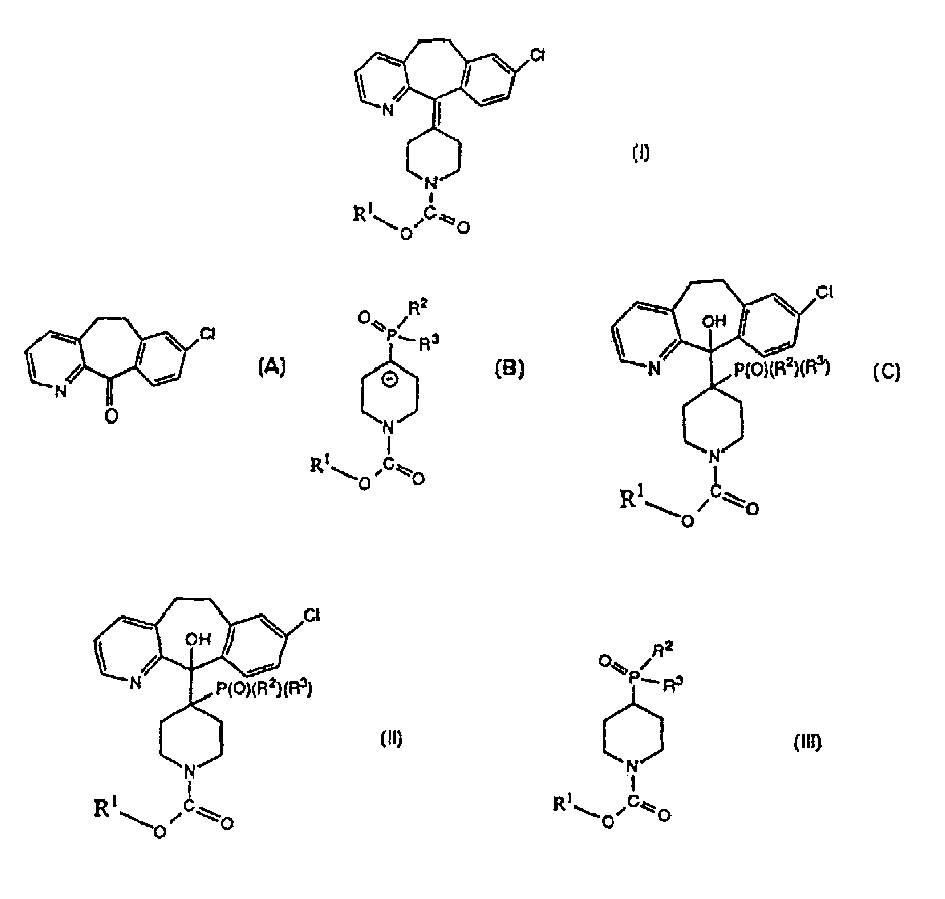
[0046] To a suspension of 3-methyl-picolinic acid (400 g; 2.92 mol) in dichloromethane ("DCM") (1600 ml) was added triethylamine (406.4 ml; 2.92 mol). The solution was cooled to -20°C and ethyl chloroformate (278.8ml; 2.92mol) was added dropwise keeping the reaction temperature between -10 and -20°C. The reaction mixture was stirred for a further 2 hours at this temperature. A solution of 4-chloroaniline (372.1 g; 2.92 mol) in dichloromethane (150 ml) was then added dropwise keeping the internal temperature between -10 and -20°C. Stirring was continued at this temperature for 2 hours, then the reaction was allowed to warm to room temperature. Water (400ml) was added to quench the reaction. The layers were separated, and the organic layer was washed with water (400ml). The reaction mixture was concentrated to about 800ml by removing the solvent in vacuo. Isopropanol (400ml) was added and 1 volume of solvent was evaporated under reduced pressure. Isopropanol (400ml) was add...
preparation example B
[0048] To a solution of amide 2 (150 g; 0.61 mol) in THF (750 ml) was added 2.5M butyllithium in hexane (486 ml; 1.22 mol) at -25°C while maintaining the internal temperature between -20 and -30°C between. The mixture was stirred at -25°C for 1 hour, then 3-chlorobenzyl chloride was added dropwise over 55 minutes, also maintaining the internal temperature between -20 and -30°C. The reaction mixture was stirred at -25°C for 1 hour and then allowed to warm to room temperature. Water (300ml) was added and the resulting mixture was stirred for 30 minutes. The layers were separated and the aqueous layer was extracted with ethyl acetate (150ml). The combined organic phases were evaporated to dryness in vacuo and the residue was crystallized from isopropanol (900ml) to give 205.5g of amide 3 (91%).
preparation example C
[0050] Add N-(4-chlorophenyl)-3-[2- (3-Chlorophenyl)ethyl]-2-pyridinecarboxamide (30g; 0.081mol) in dichloromethane (60ml). The resulting mixture was stirred at 5 to 10°C for 1 hour and then allowed to warm to room temperature over 30 minutes. Aluminum chloride (43.1 g; 0.323 mol) was added in 4 portions over 45 minutes keeping the reaction temperature below 30°C. The mixture was stirred for 1 hour then poured onto ice (300g). Dichloromethane was removed from the mixture by distillation, and the remaining aqueous solution was heated at 80°C for 1 hour. Trisodium citrate dihydrate (70 g; 0.24 mol) was added followed by aqueous sodium hydroxide (10 M, 140 ml) to adjust the pH to 7. Toluene (150ml) was added followed by a solution of maleic anhydride (12.0g 0.122mol) in toluene (50ml). The resulting mixture was stirred for 30 minutes, then the pH of the aqueous phase was adjusted to 12 with aqueous sodium hydroxide (10M, 60ml). The mixture was heated to 70°C and separated. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com