Freeze concentration for aqueous solutions
A freeze-concentration and aqueous solution technology, applied in solution crystallization, heat exchange cooling crystallization, general layout of crystallization devices, etc., can solve the problems of non-commercial application, low refrigeration efficiency, and extra energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
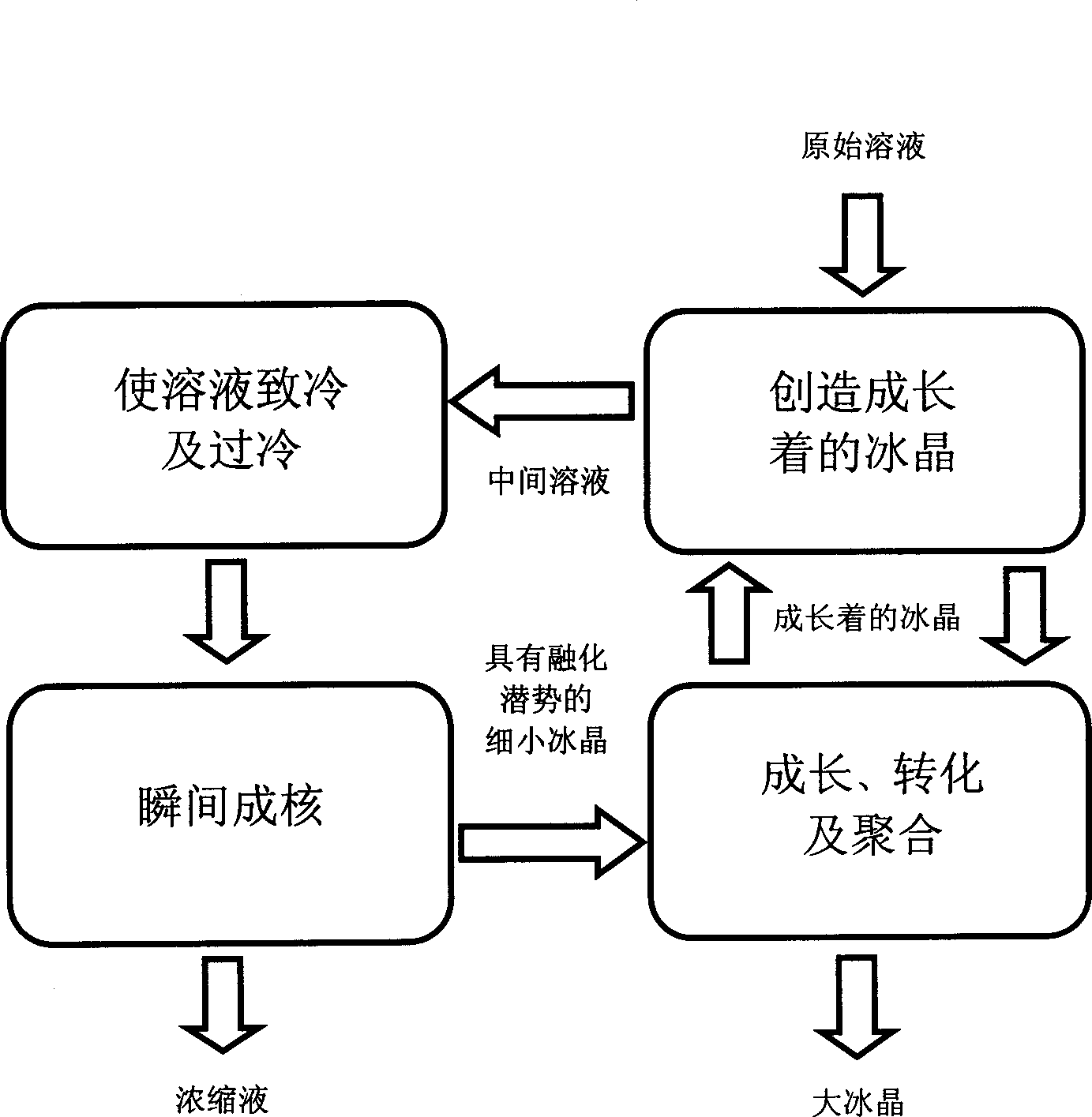
[0059] This example illustrates subcooling of an aqueous solution without nucleation in a refrigeration heat exchanger.
[0060] Sucrose solutions of varying concentrations, prepared with commercial granulated sugar and water, were cooled in laboratory-scale double-pipe heat exchangers, with the solutions in the tubes and the coolant supplied by a refrigerated water bath in the annulus, operating as countercurrent. The technique described in the "Principles of Supercooling, Metastable Solutions and Instantaneous Nucleation" in the "Best Mode for Carrying Out the Invention" section was used in the experiments to achieve higher degrees of subcooling. The results of the degree of subcooling achieved without nucleation are shown in Table I.
[0061] Table I
[0062] Solution concentration Freezing point Test times Subcooling temperature difference
[0063] (weight%) (°C) (°C) (°C)
[0064] 5 -0.34 8 3.8-6.8 1.0-1.8
[0065] 10 -0.61 9 4.4-6.7 0.8-1.6
[0066] ...
Embodiment 2
[0071] This example illustrates the growth, transformation and aggregation of ice crystals in a lab-scale (1 L) multipurpose crystallizer with cooling jacket. The sucrose solutions with different concentrations are supercooled by a laboratory-scale sleeve-and-tube heat exchanger. The supercooled solution is seeded and nucleated, and the resulting fine ice crystals are separated from the concentrated solution by a filter. For the growth, transformation and aggregation of ice crystals, the obtained fine ice crystal slurry is added to a multifunctional crystallizer containing a suspension of large growing ice crystals. As a starting solution, a 5% sucrose solution was also added to the crystallizer. The mean values and standard deviations of the results obtained through several experiments under different conditions are listed in Table II.
[0072] Table II
[0073] Concentration Range Low Medium Low Medium High High
[0074] Number of trials 4 6 4 6
[0075] C r (we...
Embodiment 3
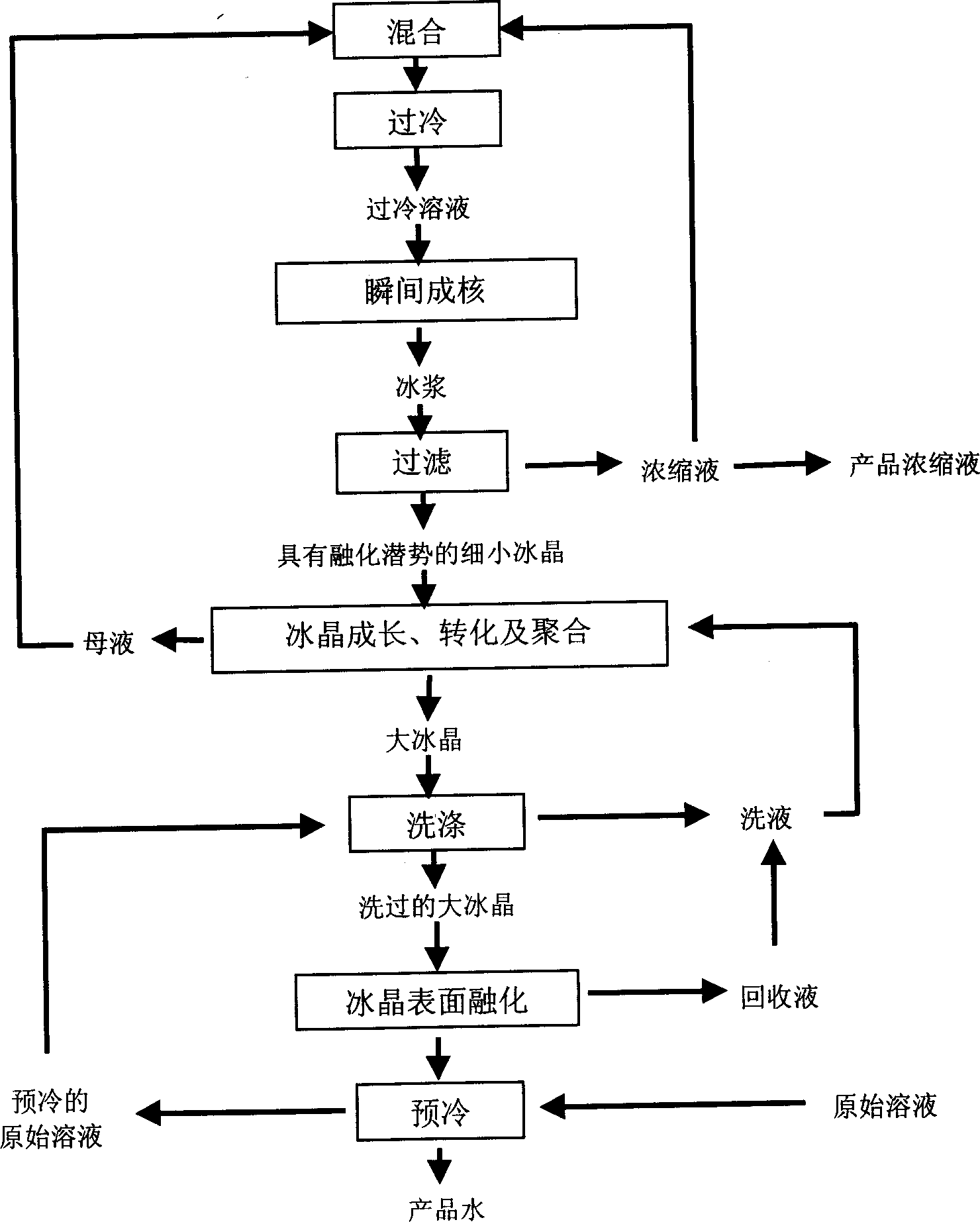
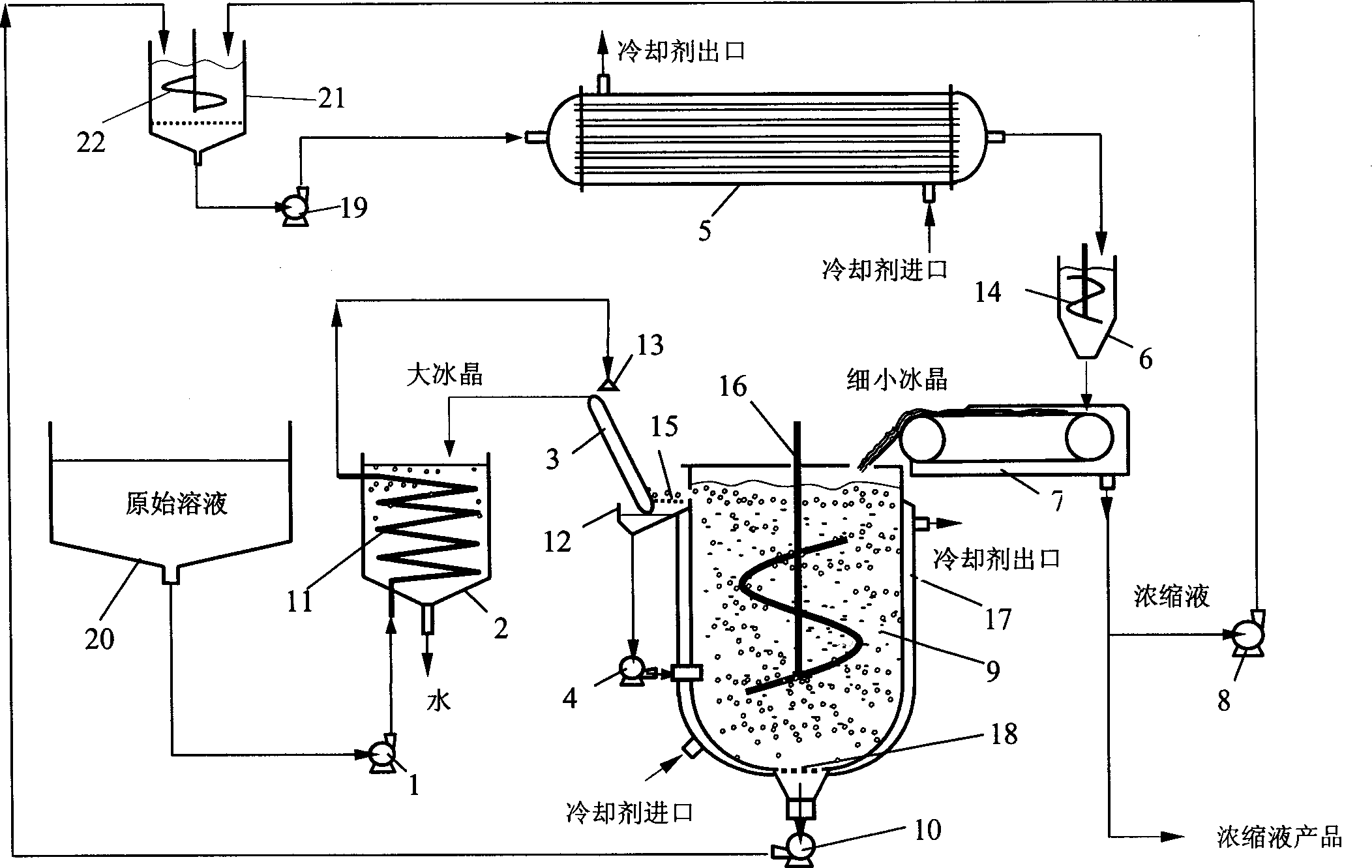
[0095] This example illustrates a three-stage freeze concentration process for a sucrose solution, operated according to Figure 5 The process shown is carried out. The material balance and operating parameter data of the three freeze concentration stages and the two step combinations of the three-stage freeze concentration process are given in Table III and Table IV, respectively. The mass balance is based on 100 kg of the 5% original sucrose solution to be treated.
[0096] The meanings of the symbols in Table III and Table IV, except those already stated in Table II, are as follows:
[0097] W r - Mix the weight of the sucrose solution in the refrigerated heat exchanger.
[0098] W ri - the weight of the sucrose solution mixed with the circulating solution.
[0099] W rc - the weight of the circulating solution.
[0100] W fc - the weight of the concentrate after separation from the fine ice crystal slurry.
[0101] W fi - the weight of the ice in the fine ice cryst...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com