Ethene oligomerization and polymerization post transition metal complex catalyst
A late transition metal, coordination catalyst technology, applied in the directions of organic chemistry, hydrocarbons, hydrocarbons, etc., can solve the problems of complex synthesis steps and preparation methods, and achieve easy mass production, simple preparation, and good stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
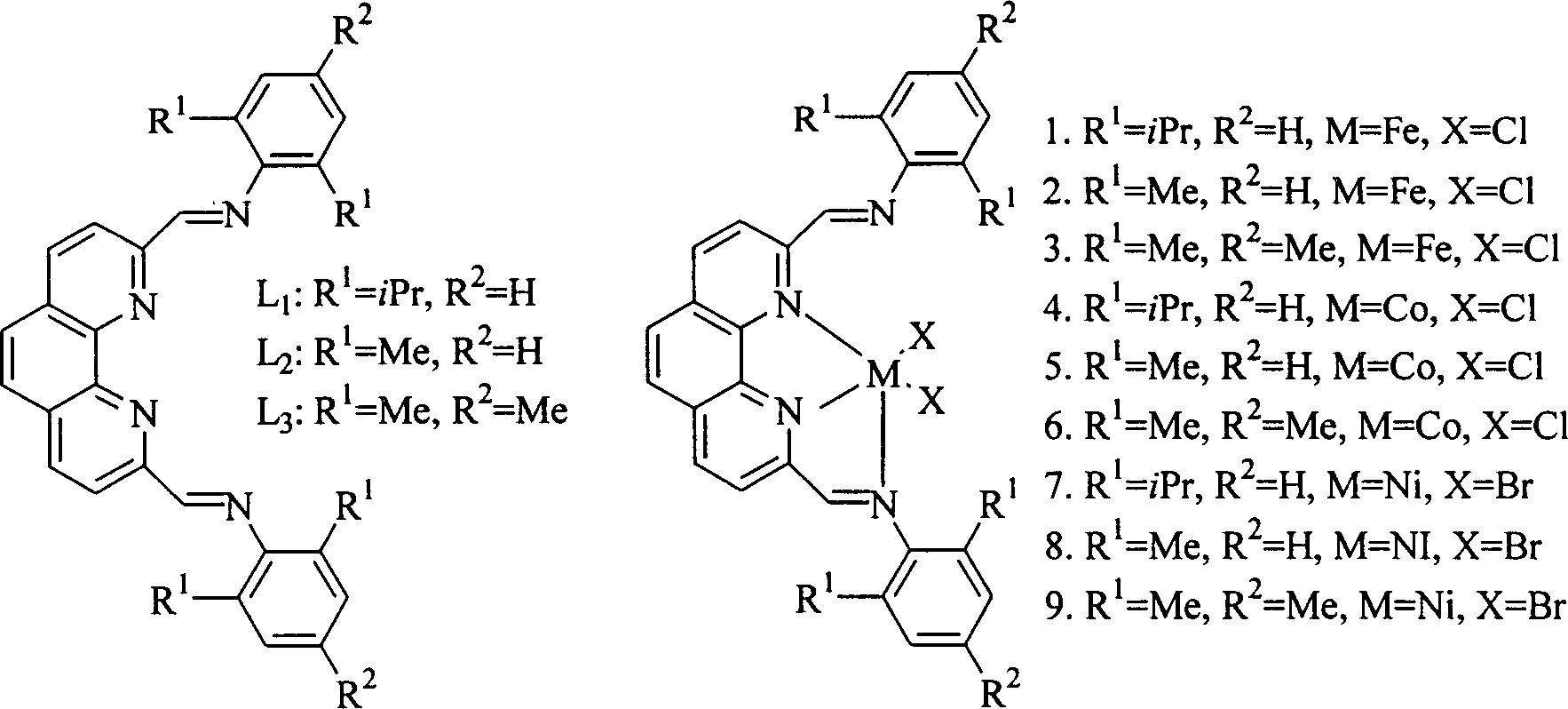
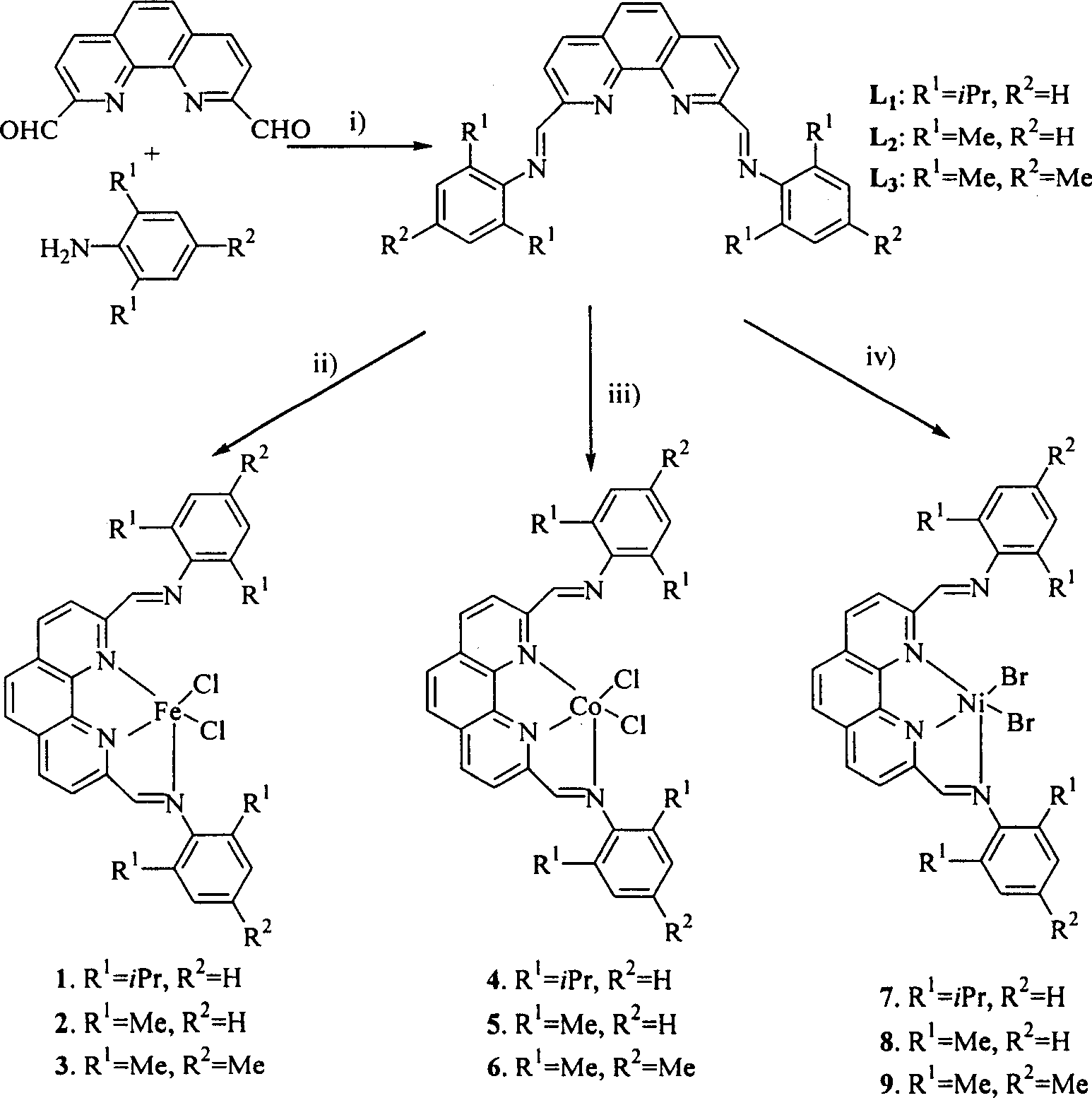
[0018] 2,9-bis(2,6-diisopropylphenylimino)-1,10-phenanthroline (L 1 ) preparation of catalyst:
[0019] Under nitrogen protection, 2,9-diformyl-phenanthroline (1.0g, 4.2mmol) was dissolved in absolute ethanol solution (120mL), then 3 drops of acetic acid were added, then the temperature was raised to reflux, and stirred for half an hour A solution of 2,6-diisopropylaniline (4.0 g, 20 mmol) in absolute ethanol (15 mL) was added dropwise. After the dropwise addition, continue to stir and reflux for 5 hours, cool to room temperature, and evaporate most of the solvent under reduced pressure. The product mother liquor is placed in the refrigerator overnight, and 1.56 g of yellow crystals are precipitated, yield: 62%. m.p.269-270℃; IR(KBr): 3063(s), 2962(s), 1639(vs), 1586(s), 1550, 1499, 1462(m), 1383, 1363, 1251, 1182, 1086, 933(m), 860, 756(s). 1 H NMR (CDCl 3 ): δ1.18 (24H, d, J = 10.2Hz), 3.05 (4H, m), 7.15-7.23 (6H, m), 7.99 (2H, s), 8.47 (2H, d, J = 7Hz), 8.75 (2H, d, J=...
Embodiment 2
[0025] Ligand 2,9-bis(2,6-dimethylphenylimino)-1,10-phenanthroline (L 2 ) preparation: utilize synthetic ligand 2 in the embodiment 1, 9-two (2,6-diisopropylphenylimino)-1,10-o-phenanthroline (L 1 ) method, 2,9-diformyl o-phenanthroline and 2,6-dimethylaniline react to obtain yellow solid 2,9-bis(2,6-dimethylanilimino)-1,10 -O-phenanthroline, yield: 58%.m.p.132-133°C.IR (KBr):3449m 3325(m), 3018(m), 2969,2918(m), 1636(vs), 1591,1550( s), 1500, 1474, 1442(s), 1192, 1088(s), 861, 762(s). 1 H NMR (CDCl 3 )δ2.18 (12H, s), 6.94-7.14 (6H, m), 8.05 (2H, s), 8.42-8.52 (2H, d), 8.75-8.86 (4H, m); EI-MS (m / z): 442 (M + , 100%), 441 (39.5%), 427 (14.9%), 424 (16.7%), 337 (28.4%), 324 (18.2%), 323 (53.2%), 322 (14.1%), 311 (25.2 %), 310 (64.7%), 309 (14.3%), 308 (11.4%), 296 (14.1%), 295 (10.5%), 132 (13.5%); Anal. Calcd. For C 30 h 26 N 4 1 / 2H 2 O: C, 79.79; H, 6.03; N, 12.41. Found: C, 79.86%; H, 6.41; N, 12.13%.
[0026] Preparation of 2,9-bis(2,6-dimethylphenylimino)-1,10-p...
Embodiment 3
[0030] Ligand 2,9-bis(2,4,6-trimethylphenylimino)-1,10-phenanthroline (L 3 ) preparation: Utilize the synthetic ligand 2,9-bis(2,6-diisopropylphenylimino)-1,10-phenanthroline (L 1 ) method, 2,9-diformyl-phenanthroline and 2,4,6-trimethylaniline were reacted to obtain a yellow solid, yield: 77%. m.p.166-167℃; IR(KBr): 3394(br), 1724(s), 1633(vs), 1553(m), 1499, 1480(s), 1208(s), 854(s); 1 H NMR (CDCl 3 ): δ2.06(12H, s), 2.23(6H, s), 6.85(4H, m), 7.90(2H, s), 8.38(2H, d); 8.43-8.86(4H, m); FAB- MS (m / z): 471 (M + +1);Anal.Calcd.For C 32 h 30 N 4 h 2 O·H 2 O: C, 78.66; H, 6.60; N, 11.47. Found: C, 78.29%; H, 6.31; N, 11.06%.
[0031] Preparation of 2,9-bis(2,4,6-trimethylbenimino)-1,10-phenanthroline iron complex (3): Synthesis of 2,9-bis( 2,6-diisopropylphenylimino)-1,10-phenanthroline iron method, using the ligand 2,9-bis(2,4,6-trimethylphenylimino)- 1,10-Phenanthroline and FeCl 2 4H 2 O reaction gives green solid, yield: 91% yield.m.p. > 320 ℃.IR: 1610 (s), 1570 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com