Production method of imidacloprid
A production method and imidacloprid technology, which are applied in the fields of botanical equipment and methods, biocides, plant growth regulators, etc., can solve the problems of complex structure of stirred tank reactor, insufficient reaction, uneven mixing of materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] The present invention will be further described below in conjunction with embodiment:
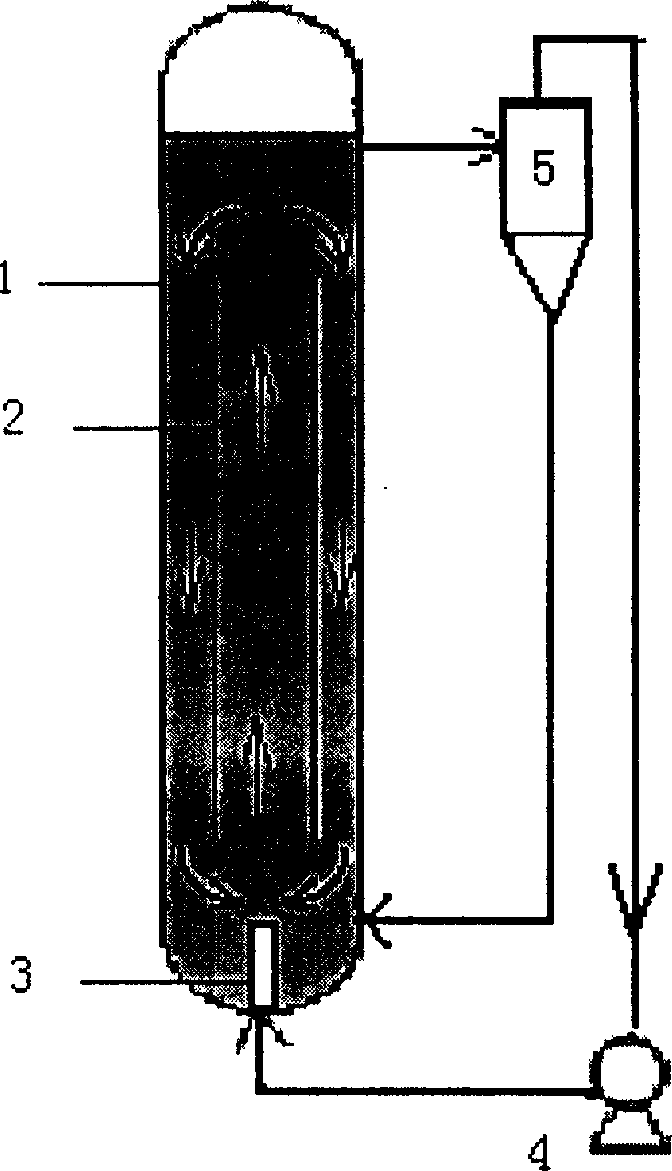
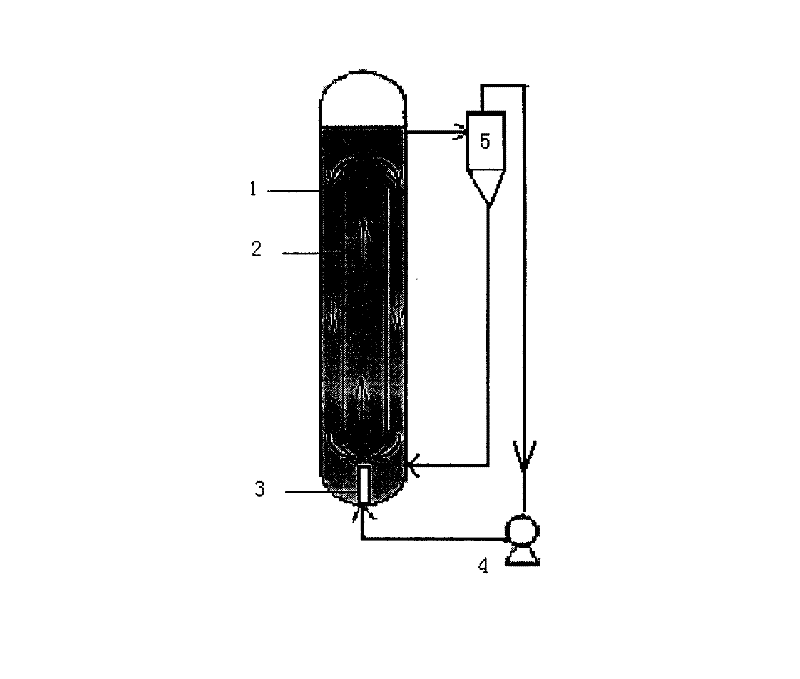
[0015] 1. Add N-nitroimino imidazolidine, potassium carbonate, tetramethylammonium hydroxide with a mass ratio of 1.13: 2.32: 0.005 in the jet loop reactor, tetramethylammonium hydroxide is a catalyst, add butanone Make solvent, make the mass / volume ratio of N-nitroiminoimidazolidine and methyl ethyl ketone be 1.13: 1, open jet pump 4 of jet loop reactor 1, under the impetus of jet pump 4, material and solvent are sprayed The circulating flow in the loop reactor 1 flows upward in the guide tube 2, and flows downward in the annular gap between the guide tube 2 and the jet loop reactor 1. The mixture of the overflow material and the solvent passes through the cyclone separator 5 to complete the solid-liquid After separation, the solid material returns to the jet loop reactor 1 from the bottom of the cyclone separator 5, and the liquid material is sprayed into the jet loop reactor 1 aga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com