Androgen receptor suppressors in treatment of hirsutism,acne and androgenetic alopecia
A technology of compounds and mixtures, applied in the field of androgen receptor inhibitors in the treatment and diagnosis of prostate cancer, alopecia and other androgen excess syndromes, can solve the problem of harmless long-term systemic treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
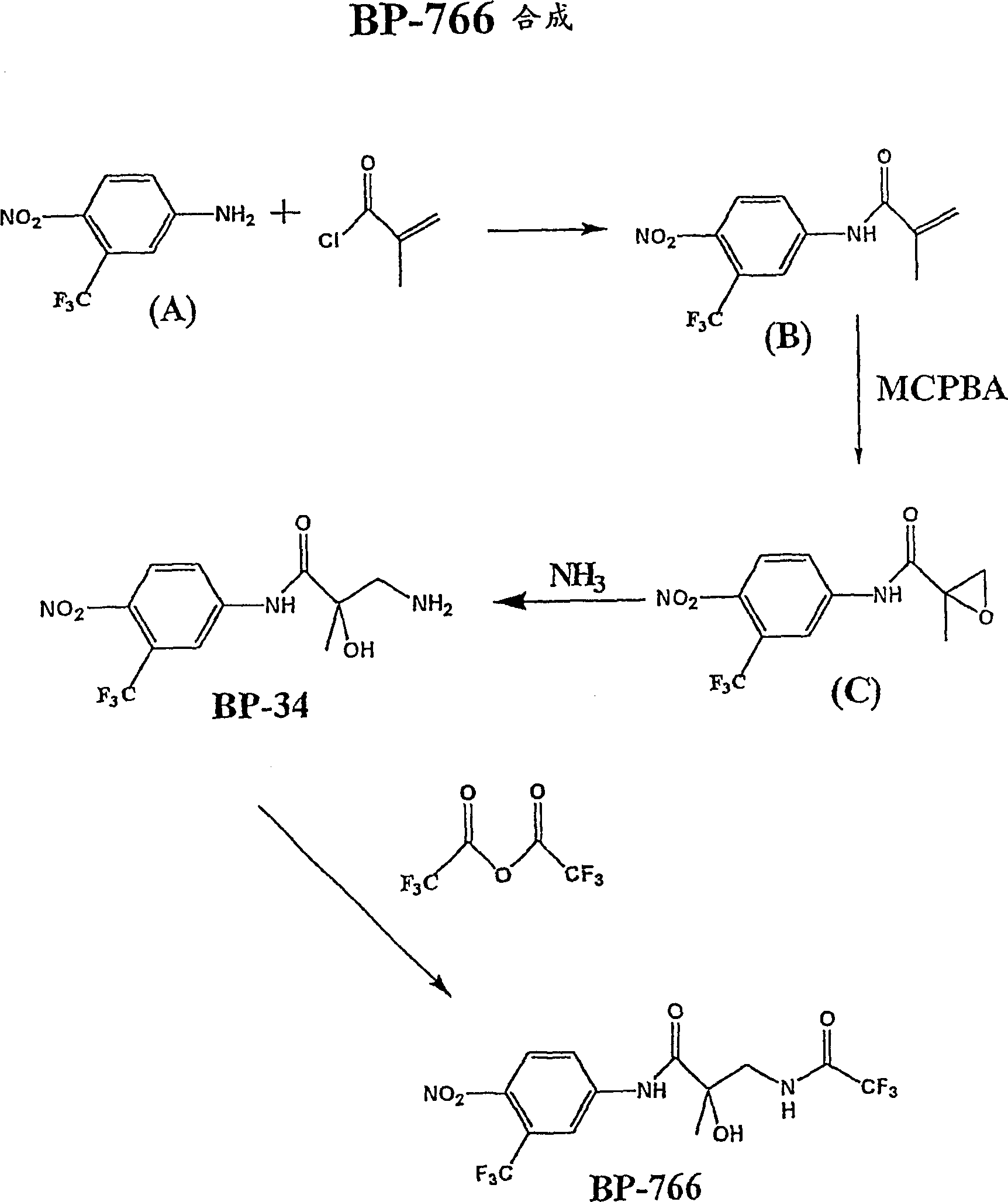
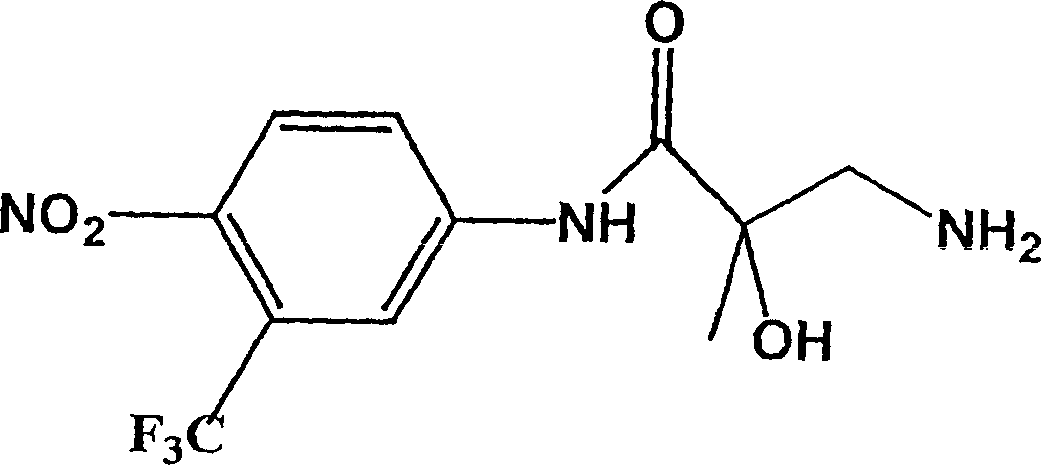
[0071] Example 1: 4-nitro-3-trifluoromethyl-N-(2-hydroxy-2-methyl-3-amino-propionyl)aniline, (BP-34)
[0072] Add 4-nitro-3-trifluoromethyl-[2,3-epoxy-2-methylpropionyl]aniline, BP-33 (10.0g, 34.46mmol) (see image 3 ) And methanol (100ml). After cooling to 70°C, excess ammonia was condensed into the reactor, which was sealed and stirred for 14 hours. After evaporation, use cold CH for the crude solid 2 Cl 2 (50ml) Wash. It was filtered and dried to obtain 6.1 g of BP-34 (yield 58%).
[0073] Melting point: 142-145°C
Embodiment 2
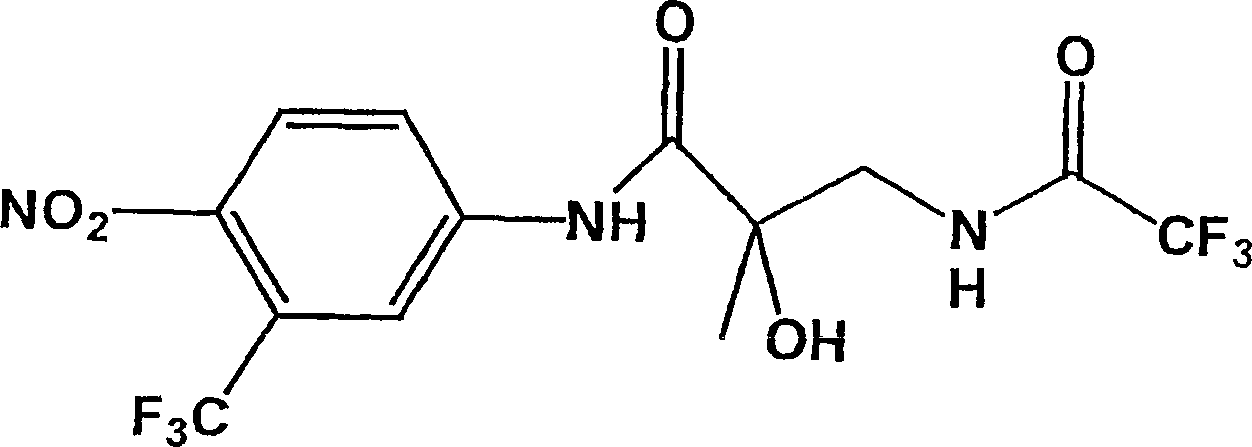
[0074] Example 2: 4-Nitro-3-trifluoromethyl-N-(2′-hydroxy-2′-methyl-3′-N-(hexafluorobutyramide Yl)propionyl)aniline, (BP-521)
[0075] BP-34 (247mg, 0.80mmol) and CH under nitrogen 2 Cl 2 (5mL), THF (10mL) and NEt 3 (1.1 mL, 0.80 mmol) was cooled to 0°C and heptafluorobutyryl chloride (120 μl, 0.80 mmol) was added. After cooling to room temperature, remove the volatiles and add CH 2 Cl 2 (30mL) and H 2 O (50mL), separate the organic layer and use MgSO 4 dry. After silica gel chromatography (CHCl 3 / Acetone) The product was isolated after purification, which was a colorless oil (320 mg, yield 82%).
Embodiment 3
[0076] Example 3: 4-Nitro-3-trifluoromethyl-N-(2'-hydroxy-2'-methyl-3'-pentafluorooctanoylamino)- Propionamide, (BP-562)
[0077] To BP-34 (360mg, 1.17mmol) was added THF (10mL) and NEt 3 (485 μL, 3.5 mmol). The solution was cooled to 0°C and pentadecyl octanoyl chloride (295 μL, 1.17 mmol) was added. After reaching room temperature, the volatiles were removed. After silica gel chromatography (CHCl 3 After purification with acetone), the product was obtained as a pale yellow solid (689 mg, yield 84%).
[0078] Mass spectrum (m / z): 704 (MH + ); 726(M+Na + ). 19 F NMR(470MHz, CDCl 3 ): -56.8 ppm, -77.3, -116.3, -118.1, -118.6, -119.1, -119.4, -122.7.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com