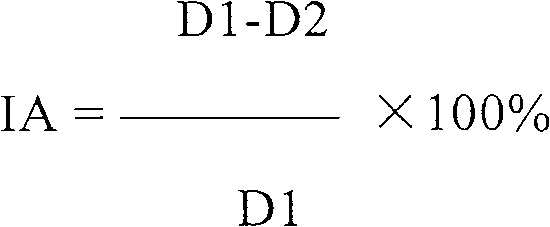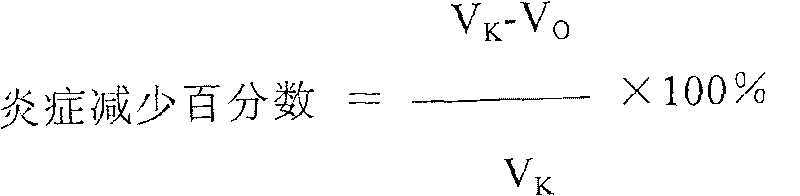Compound, composition and method for the treatment of inflammatory and inflammatory-related disorders
A composition and drug technology, applied in the direction of drug combination, anti-inflammatory agent, pharmaceutical formula, etc., can solve the problem of small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Embodiment 1. The method for preparing yeast RNA
Embodiment 11
[0104] Example 1.1 Production of Yeast RNA
[0105]Preparation of RNA-D from S. cerevisiae and RNA-P, RNA-PN and RNA-F from Candida utilis. Yeast RNA extraction was carried out at 100-110°C with 10-12% sodium chloride solution. The RNA solution was isolated from the yeast pellet, cooled to 0 °C and acidified to pH 1-2 with HCl. Precipitated RNA was rinsed with alcohol, dried and dissolved in water. The solution was adjusted to pH 8.0-8.2 with sodium hydroxide. Place at 37-40°C for about 1 hour after adding trypsin. Heat to inactivate the enzyme, then filter the solution. RNA was precipitated from cold alcohol, acidified to pH 1-2 with hydrochloric acid, and dried. RNA-F was prepared in this way. Further, the precipitate was filtered, rinsed with alcohol, and then dissolved in water adjusted to pH 6.2-6.5 by adding sodium hydroxide. RNA-PN was precipitated by ethanol. The precipitate was filtered and dried. RNA-P was derived from RNA-F by repurification from protein by...
Embodiment 12
[0110] Example 1.2. No toxicity
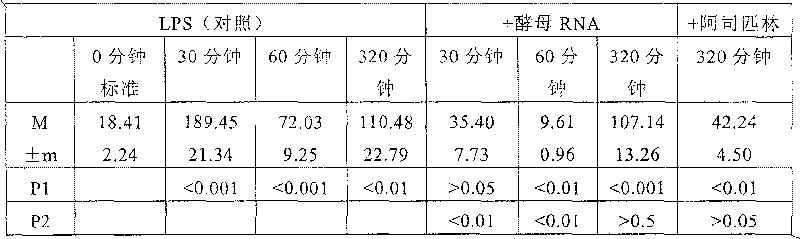
[0111] We confirmed the non-toxicity of yeast RNA-P and RNA-D. Intraperitoneal administration of single or multiple doses of yeast RNA in biologically active amounts (250-500 mg / kg body weight) did not result in substantial changes in the number of peripheral lymphocytes in mice. These changes would be characteristic indicators of endotoxin.
[0112] Similar results were obtained with intravenous introduction of nucleic acids. We measured changes in the amount of peripheral leukocytes 1-3 hours after intravenous injection of 100 mg of yeast RNA-P or RNA-D solution in rabbits. An intravenous injection of 0.85% NaCl solution was used as a non-toxicity standard. The experimental results proved that, similar to the standard, the injection of yeast RNA-P or RNA-D did not induce changes in the number of white blood cells within 3 hours of introduction. In animals administered 0.85% NaCl solution, the amount of leukocytes was equal to 13000±980, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com