Pharmaceutical composition comprising pyrroloquinoline quinone for curing and preventing fatty liver
A technology of quinoline quinone and fatty liver, which is applied in the medical field and can solve the problems that the drugs for fatty liver cannot achieve results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
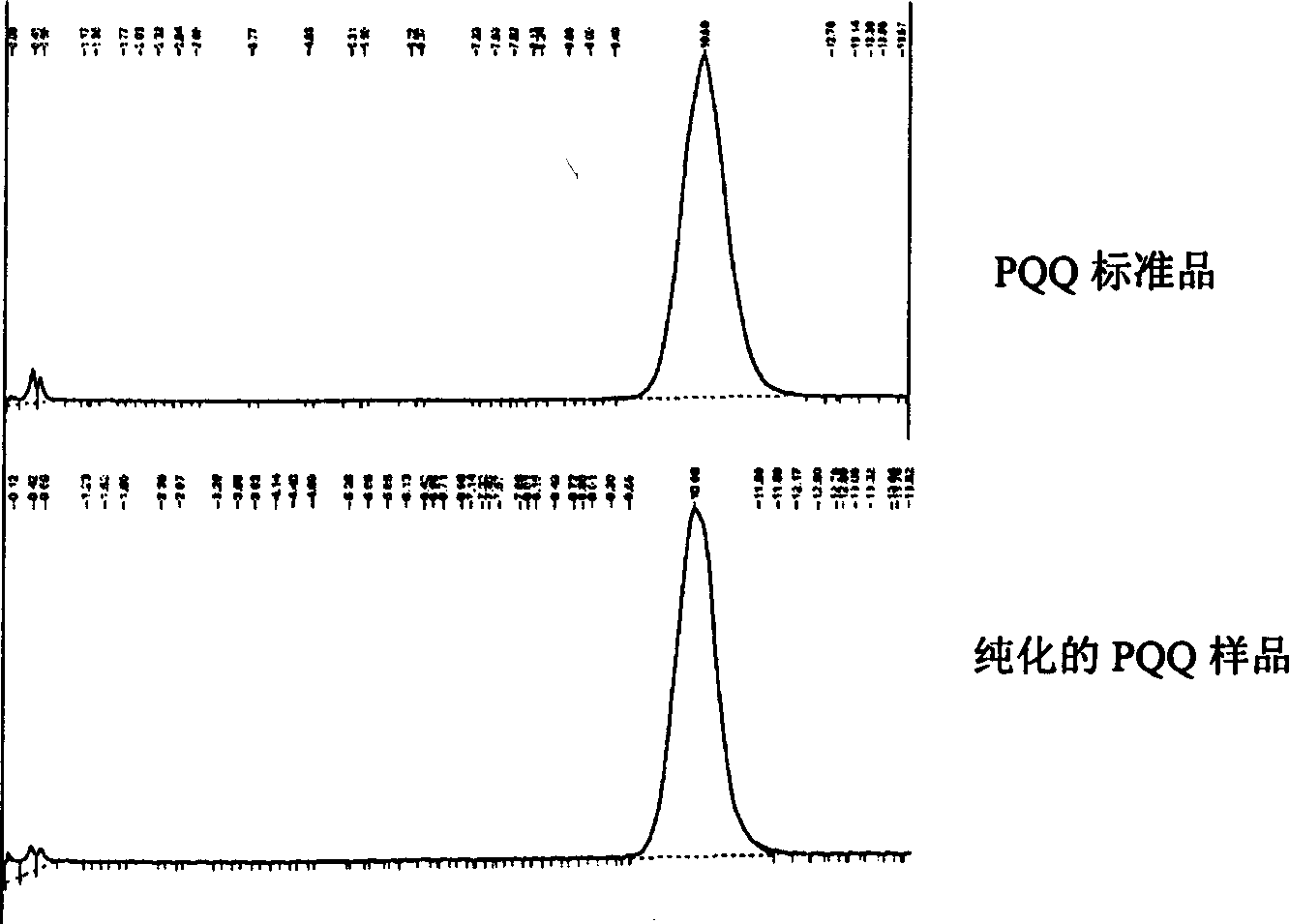
[0048] Establishment of a method for the detection of pyrroloquinoline quinone
[0049] In the present invention, two methods of NBT chromogenic method and FPLC are used to detect the content of pyrroloquinoline quinone.
[0050] The NBT method uses pyrroloquinoline quinone to make NBT (nitro blue tetrazolium chloride) chemical color development in alkaline solution, and can quantitatively detect the content of pyrroloquinoline quinone in the mixture (more than 10ng can be detected pyrroloquinoline quinone).
[0051] The specific operation is as follows: add 400ul water containing 25ul bovine serum albumin (40mg / ml) to the pyrroloquinoline quinone sample or standard substance to be tested, add 1.6ml assay buffer (NBT+1.6M glycine buffer) for each sample, Mix well. Incubate at 37°C for 45 minutes in the dark, and measure the light absorption value at 530 nm. Make standard curve accordingly, calculate the content of pyrroloquinoline quinone in the sample to be tested.
[005...
Embodiment 2
[0054] Preparation of pyrroloquinoline quinone
[0055] A. Fermentation of pyrroloquinoline quinone
[0056] A common method for obtaining pyrroloquinoline quinone is to use methanolophilic bacteria to synthesize pyrroloquinoline quinone, and obtain pyrroloquinoline quinone by fermentation and separation. Strains such as Methylophagathalassica (ATCC #33145) can be used directly, or improved strains provided by PQQ production can also be used. The specific method is as follows.
[0057] Methylophaga thalassica (ATCC#33145), by changing the concentration of methanol (0.1-7%), screened a strain, the expression of PQQ from 0.5mg / L to 2.0mg / L, this strain was named GCpqq303.
[0058] Training time
(sky)
PQQ output
(mg / L)
FeCl 3 content
(%)
PQQ output
(mg / L)
MgSO 4 content
(%)
PQQ output
(mg / L)
1-7
≈0
0
0.04
0
0.07
8
0.3
0.005
...
Embodiment 3
[0062] Therapeutic and preventive effects of pyrroloquinoline quinone on alcoholic fatty liver
[0063] The Kunming mice were divided into 10 groups, 12 in each group, half male and half male, with an average body weight of 25 grams. The grouping situation is shown in the table below, and the specific experimental method is described as follows: Alcohol gavage is 0.25ml / day of white wine with an alcohol concentration of 56%, for 15 days, and a mouse model of acute alcoholic fatty liver is established. PQQ is divided into a treatment group and a prevention group. The treatment group is treated by intragastric administration or intraperitoneal injection for 30 days after the fatty liver model is established; PQQ was carried out for 30 days to determine the preventive effect of PQQ on fatty liver.
[0064] Group No
group name
illustrate
1
Fatty liver model
alcohol gavage
2
PQQ treatment group
Fatty liver model, intraperitoneal in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com