Method for carbamoylating alcohols
A kind of technology of alcohol carbamoyl and methanesulfonic acid, applied in the field of carbamoylation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Love and Kormendy's method
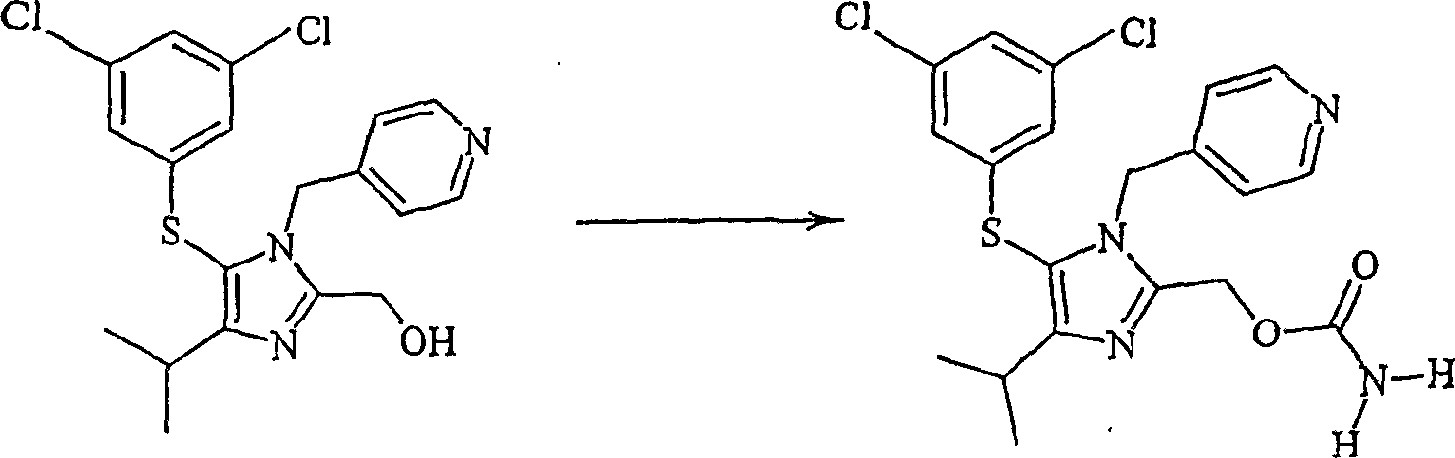
[0040] Loev and Kormendy (supra, 1963) used trifluoroacetic acid and sodium cyanate for carbamylation of alcohols. Studies of the carbamoylation of alcohols containing a thiocarbyl group and a basic moiety were initiated with these reagents.
[0041] Materials and methods
[0042] Capravirine thiols
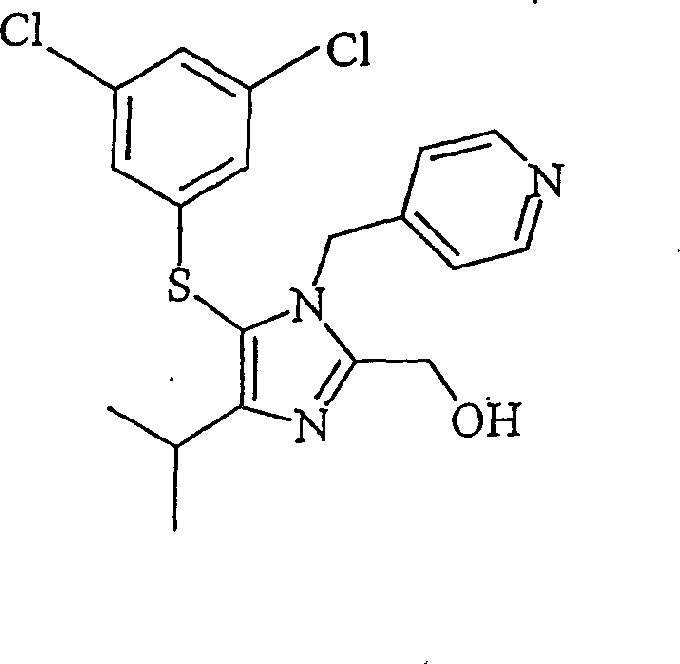
[0043] In this study, the alcohol was the thiol precursor of the antiviral agent Capravirine. Capravirine hydrocarbon thiols are prepared by:
[0044] Benzyloxy((4-isopropyl-1-(4-pyridyl)methyl)-1H-imidazol-2-yl)-hydrogen oxalate (171.6 g, 0.342 moles) in toluene (460 mL) and water (150 mL) to make a slurry. The mixture was stirred and cooled to below 10°C. The mixture was slowly cooled to a final pH of 11-12 by adding 32% aqueous potassium hydroxide (KOH). The organic layer was separated and washed with water and then saturated sodium chloride solution. Benzyloxy((4-isopropyl-1-(4-pyridyl)methyl)-1H-imidazol-2-yl)-methane in toluene...
Embodiment 2
[0052] Carbamylation of alcohols with sodium cyanate in the presence of sulfuric acid or acetic acid
[0053] The carbamoylation of alcohols containing thiol and base moieties with sodium cyanate was studied in the presence of sulfuric or acetic acid.
[0054] Materials and methods
[0055] Capravirine mercaptans were obtained as described in Example 1 above. Also as described in Example 1, the other reactants were all the finest commercial grades and were used without further purification.
[0056] The conversion of Capravirine thiols to Capravirine was carried out as described in Example 1 with the difference that sulfuric acid or acetic acid was used instead of TFA. Therefore, the slurry was cooled to -10°C, then sulfuric acid (18 mL), or acetic acid (30 mL) was added dropwise while keeping the temperature below 0°C.
[0057] result
[0058] Sulfuric acid and acetic acid provided higher yields than TFA. The conversion of carbamylated alcohols in this process was 1...
Embodiment 3
[0060] Carbamylation of alcohols with sodium cyanate in the presence of methanesulfonic acid
[0061] The carbamoylation of alcohols with sodium cyanate was studied under various reaction conditions in the presence of acid to arrive at a suitable method.
[0062] Materials and methods
[0063] Material
[0064] Capravirine mercaptans were obtained as described in Example 1 above. Also as described in Example 1, the other reactants were all the finest commercial grades and were used without further purification.
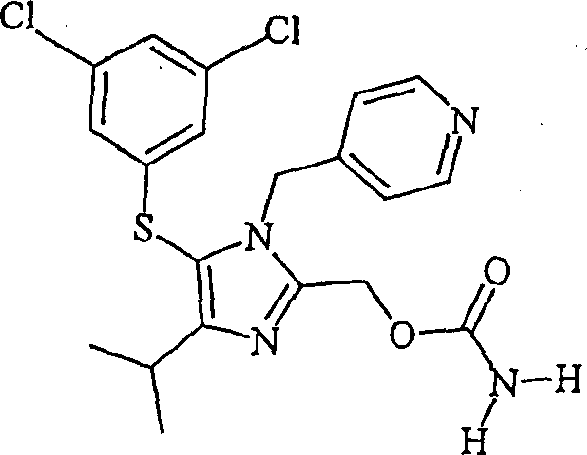
[0065] Preparation of Capravirine
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com