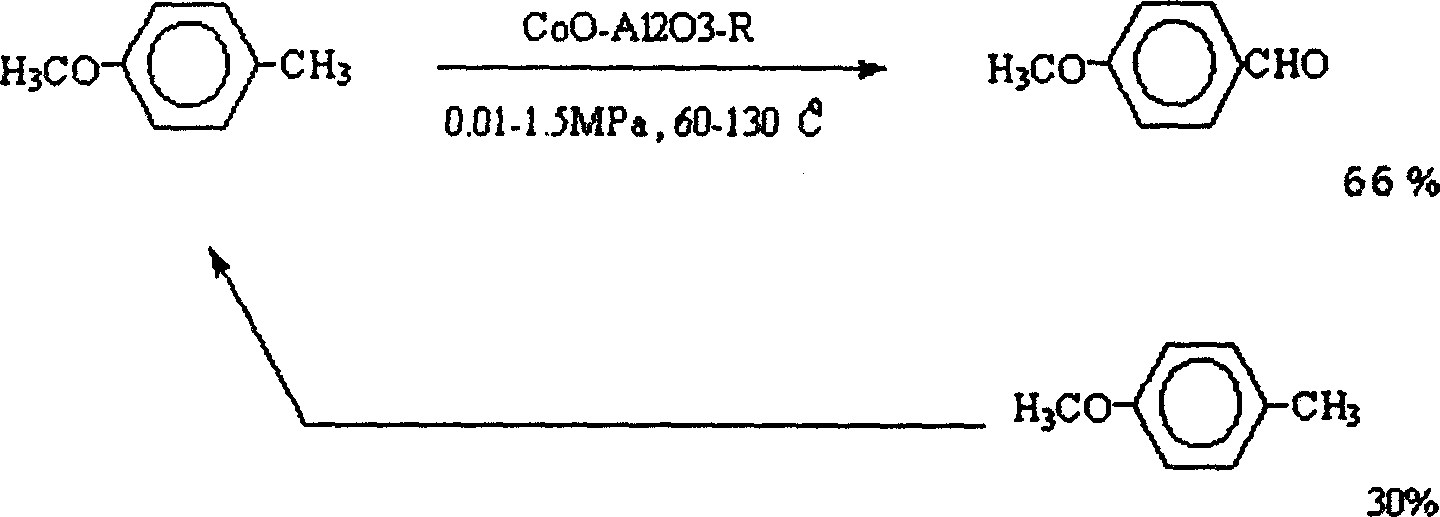
Synthetic process for p-methoxy benzaldehyde
A technology of p-methoxybenzaldehyde and p-methoxytoluene, which is applied in the field of synthesis of p-methoxybenzaldehyde, can solve problems such as environmental pollution, achieve the effect of easy separation and recovery, and avoid pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 1) Preparation of CoO-Al 2 o 3 catalyst
[0015] Dissolve 20 grams of cobalt nitrate in 100 ml of denatured alcohol, then add 30 grams of Al with a size of 15-60 nanometers 2 o 3 , soaked for 24 hours, filtered and roasted at 450°C for 4 hours to obtain the main catalyst;
[0016] 2) to buy technical grade benzoyl peroxide or azobisisobutyrocyanide as co-catalyst; present embodiment adopts benzoyl peroxide;
[0017] 3) Add 100 grams of acetic acid solvent, 30 grams of p-methoxytoluene, 0.3 grams of main catalyst, and 0.02 grams of benzoyl peroxide into an autoclave with a volume of 250 ml respectively, heat up to 100° C. under stirring, and then use oxygen Stamping to 0.6Mpa, after constant temperature reaction for 1 hour, the temperature is lowered, and the reaction stops.
[0018] GLC sampling analysis: acetic acid (80 g) was distilled off under normal pressure, and the recovery rate was 80%. P-methoxytoluene (15g) and 120 aldehydes (20g) were evaporated under r...
Embodiment 2
[0021] The difference from Example 1 is:
[0022] Preparation of CoO-Al 2 o 3 catalyst
[0023] Dissolve 20 grams of cobalt nitrate in 100 ml of denatured alcohol, then add 30 grams of Al with a size of 15-60 nanometers 2 o 3 , soaked for 36 hours, filtered and roasted at 500°C for 4 hours to obtain the main catalyst;
[0024] Add 100 grams of acetic acid, 30 grams of p-methoxytoluene, 0.1 grams of main catalyst, and 0.02 grams of azobisisobutyrocyanide into a 250ml autoclave, heat up to 100°C under stirring, and then press it to 1.0 with oxygen Mpa, after 2 hours of constant temperature reaction, the temperature was lowered, and the reaction stopped.
[0025] GLC sampling analysis: acetic acid (80 g) was distilled off under normal pressure, and the recovery rate was 80%. P-methoxytoluene (16g) and 120 aldehydes (18g) were distilled off under reduced pressure, the yield was 60%, and the total yield of p-methoxybenzaldehyde reached 86%.
Embodiment 3
[0027] The difference from Example 1 is:
[0028] Preparation of CoO-Al 2 o 3 catalyst
[0029] Dissolve 20 grams of cobalt nitrate in 200 ml of industrial alcohol, then add 80 grams of Al with a size of 15-60 nanometers 2 o 3 , soaked for 48 hours, filtered and roasted at 500°C for 4 hours to obtain the main catalyst;
[0030] Add 100 grams of acetic acid, 30 grams of p-methoxytoluene, 0.3 grams of main catalyst, and 0.015 grams of benzoyl peroxide into a 250ml autoclave, heat up to 135°C while stirring, and then press it to 1.2Mpa with oxygen , After constant temperature reaction for 1 hour, the temperature was lowered to stop the reaction.
[0031] GLC sampling analysis: acetic acid (80 g) was distilled off under normal pressure, and the recovery rate was 80%. P-methoxytoluene (15g) and 120 aldehydes (19g) were distilled off under reduced pressure, with a yield of 63.3%, and the total yield of p-methoxybenzaldehyde reached 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com