Method for preparing 1,2,4,5-benzene tetra-acid and 1,2,4,5-benzene tetra-anhydride
A technology of pyromellitic anhydride and pyromellitic acid, applied in the preparation of 1, can solve the problems of reduced oxidation catalyst activity and reduced oxidation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
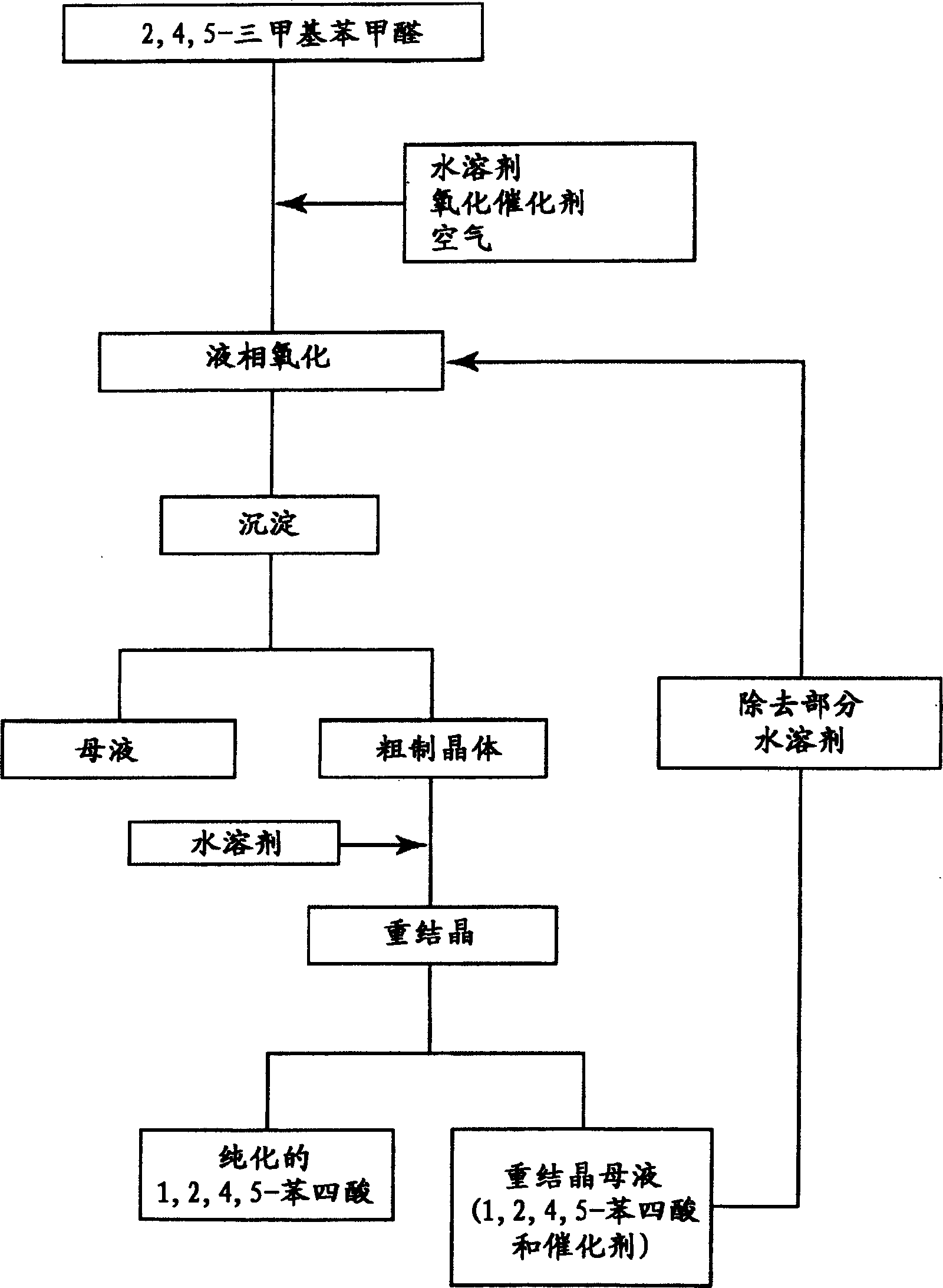
[0019] according to figure 1 In the preparation process shown, 1,2,4,5-pyrellitic acid is prepared according to the following steps.
[0020] After liquid phase oxidation of TBAL, followed by precipitation, wet crude crystals of pyromellitic acid (purity 58% by weight) were obtained. The wet crude crystals and water were fed into the recrystallization step at feed rates of 1 part by weight and 1.2 parts by weight, respectively, so that the crude crystals were redissolved in water at 120° C. and 0.2 MPa. Then, the solution was cooled to 40° C. for recrystallization. Solid-liquid separation was performed on the recrystallized slurry to obtain pure pyromellitic acid crystals, which were then rinsed with 0.8 parts by weight / hour of water. Thus, 2.1 parts by weight of a recrystallization mother liquor containing 4.6% by weight of pyromellitic acid and 0.006% by weight of manganese can be obtained per hour. After concentration, the recrystallization mother liquor was continuously...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com