Particles for inhalation having rapid release properties
A particle and rapid technology, applied in the direction of organic active ingredients, medical preparations containing non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as patient pain, reduce dosing frequency, improve coordination and comfort degree, the effect of high initial release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
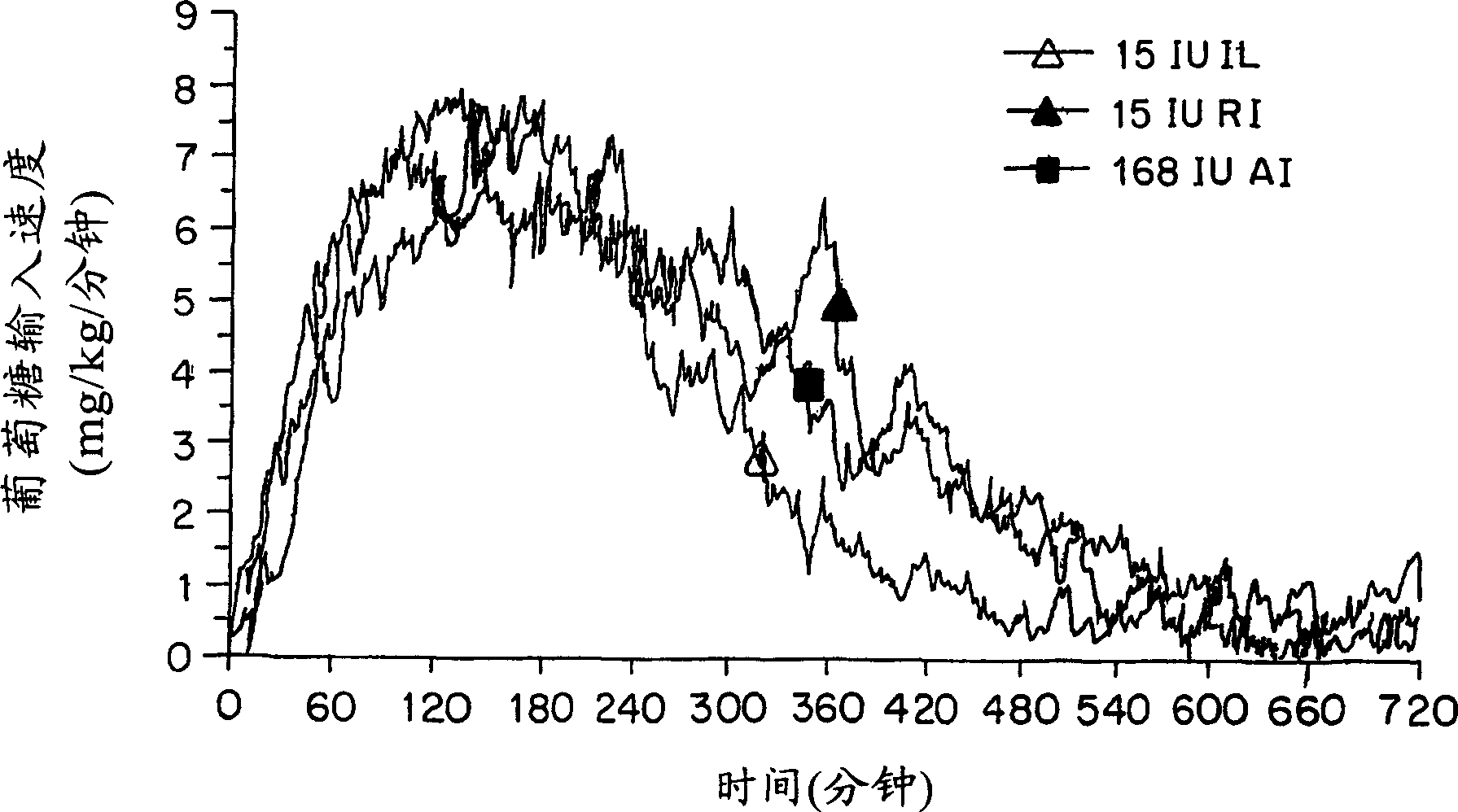
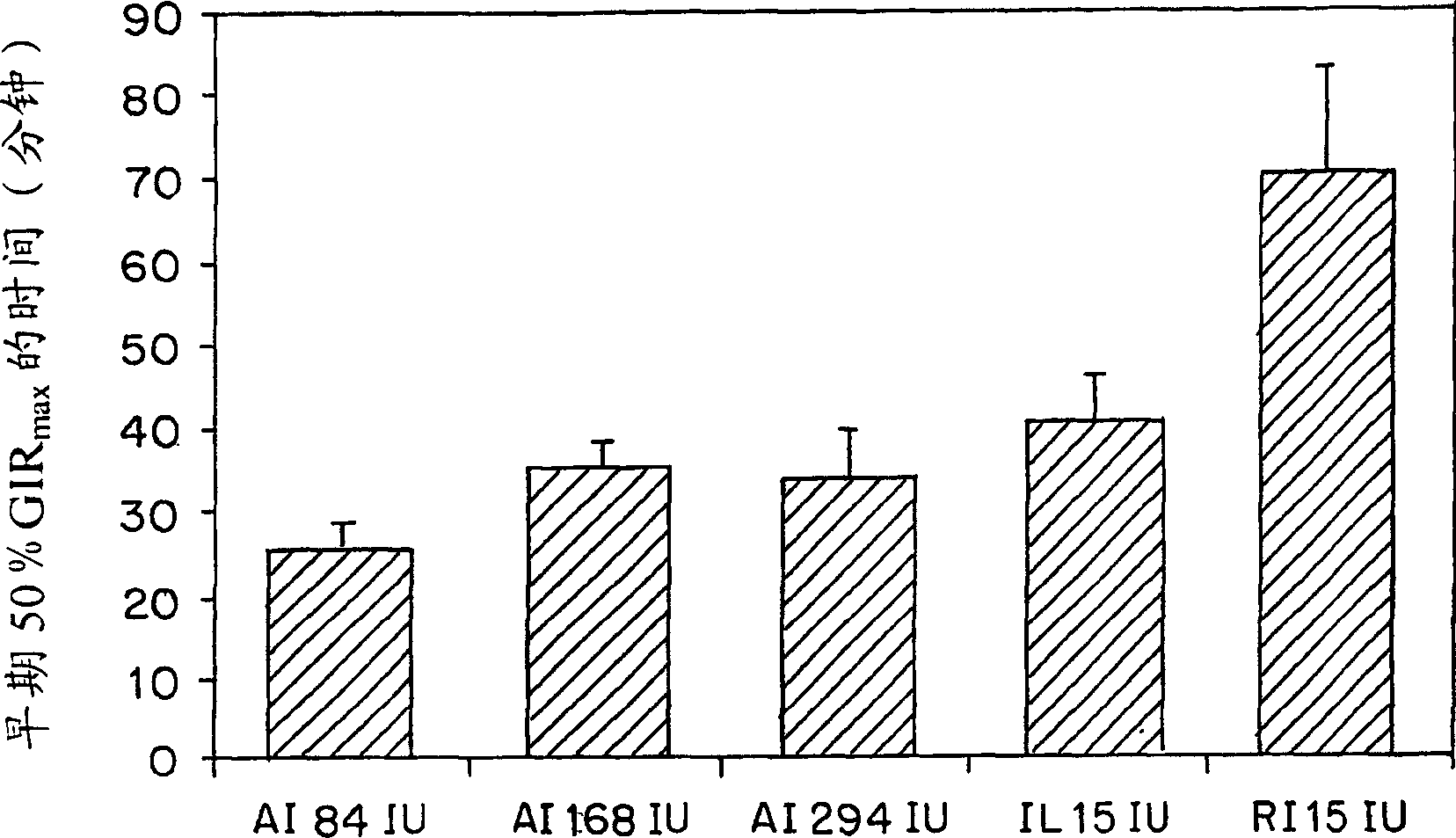
[0157] Material
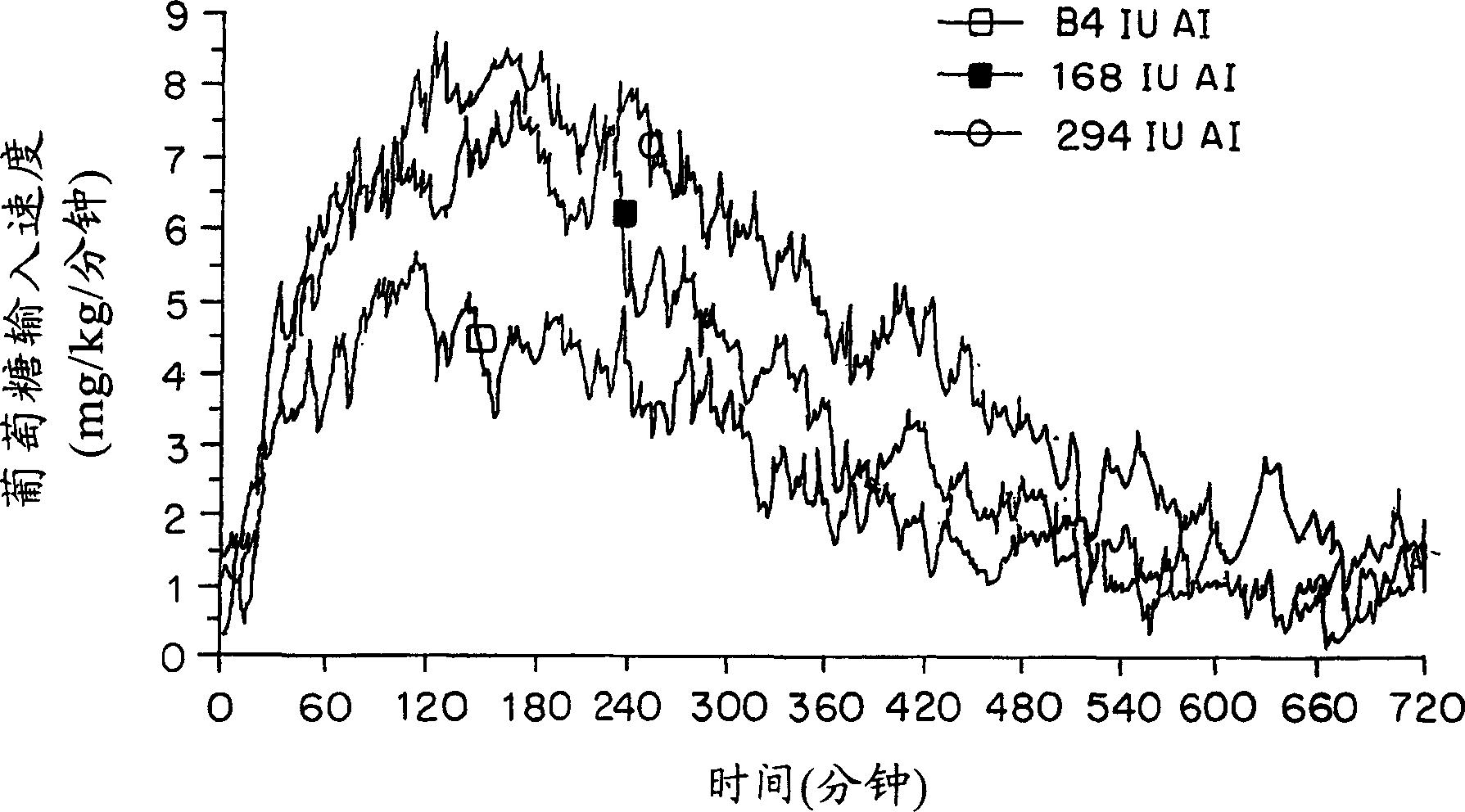
[0158] In vivo studies in rats, most of the insulin used for spray drying was obtained from BioBras (Belo Horizonte, Brazil) or Sigma (Saint Loius, MO). In in vitro studies as well as in vivo studies in humans, Humulin®, Lente® (Humulin® L human insulin zinc suspension), Humulin® R (regular soluble insulin (IR)), Humulin® Ultralente® (Humulin® U) and Humalog® 100 (insulin lispro (IL)), was obtained from Eli Lilly Company (Indianapolis, IN; 100 U / ml). These solutions were stored at 2-8°C.
[0159] Mass Median Aerodynamic Diameter - MMAD (μm)
[0160]The mass median aerodynamic diameter was determined using an Aerosizer / Aerodisperser (Amherst Process Instrument, Amherst, MA). About 2 mg of the powder formulation was added to an air disperser and the aerodynamic diameter was determined by time of flight measurement.
[0161] fine fraction
[0162] The fines fraction can be used as a way to characterize the aerosol behavior of a dispersed powder. The fine p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com