Method for composing ethoxy quinoline
An ethoxyquinoline, synthesis reaction technology, applied in the direction of organic chemistry, etc., can solve the problems of long reaction time, low conversion rate per pass, low conversion rate, etc., and achieve low cost of raw materials, high conversion rate per pass, and fast reaction speed. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
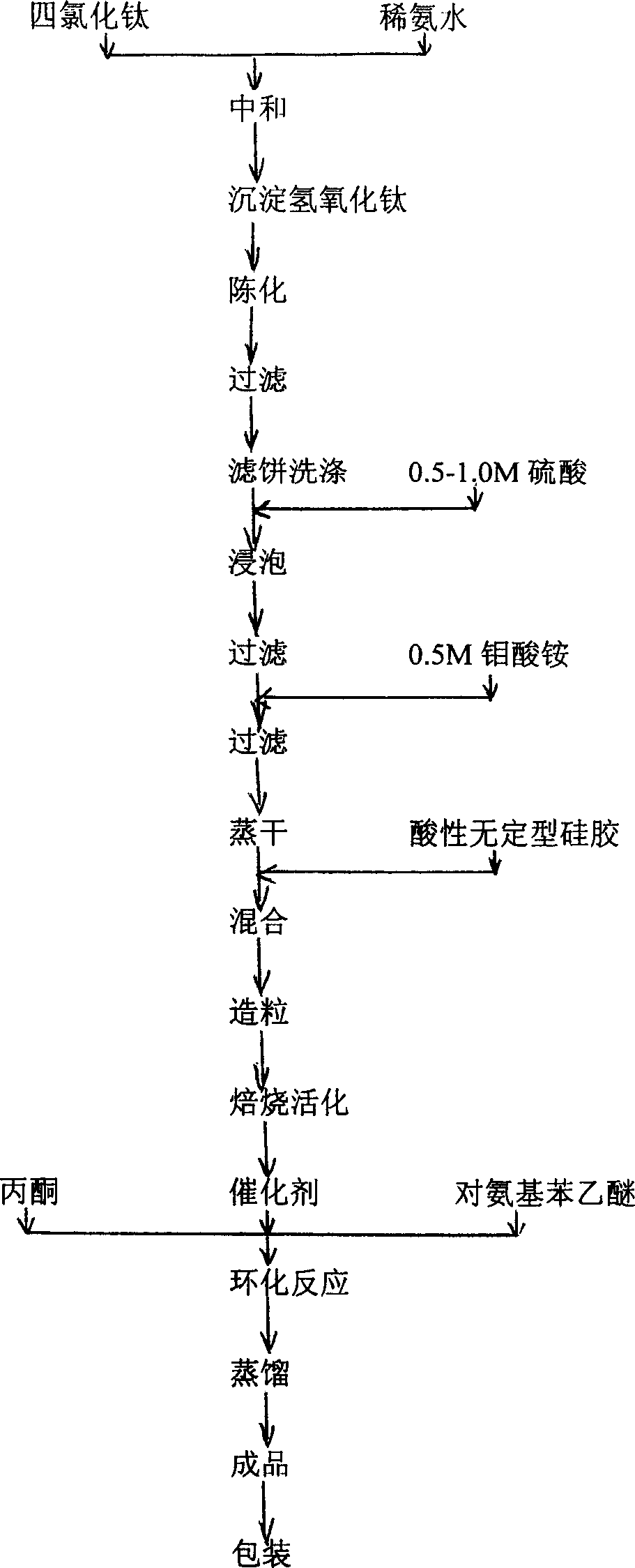
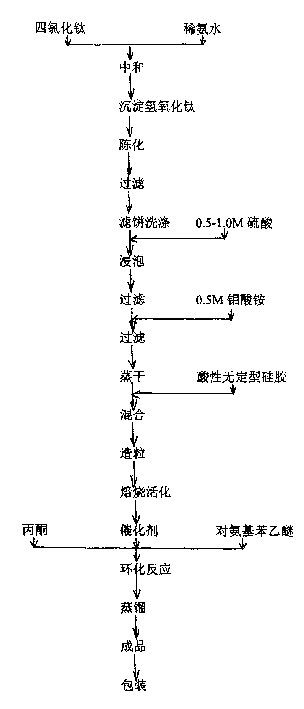
[0015] Catalyst manufacture: TiCl 4 Dissolve in ice water, neutralize with dilute ammonia water to PH=9-10 under cooling, age the resulting precipitate for 10 hours, filter, wash the filter cake, and dry at 110°C for 24 hours to obtain Ti(OH)4; take Ti( OH)4 powder, with 0.5-1M H 2 SO 4 Soak for 20 minutes, filter, then soak in 0.5M ammonium molybdate solution for 60 minutes, evaporate to dryness at 110-120°C, add acidic amorphous silica gel and stir until it can be granulated, and shape it to 20-30 mesh by a granulator, 500 The catalyst was calcined at ℃ for 3 hours.
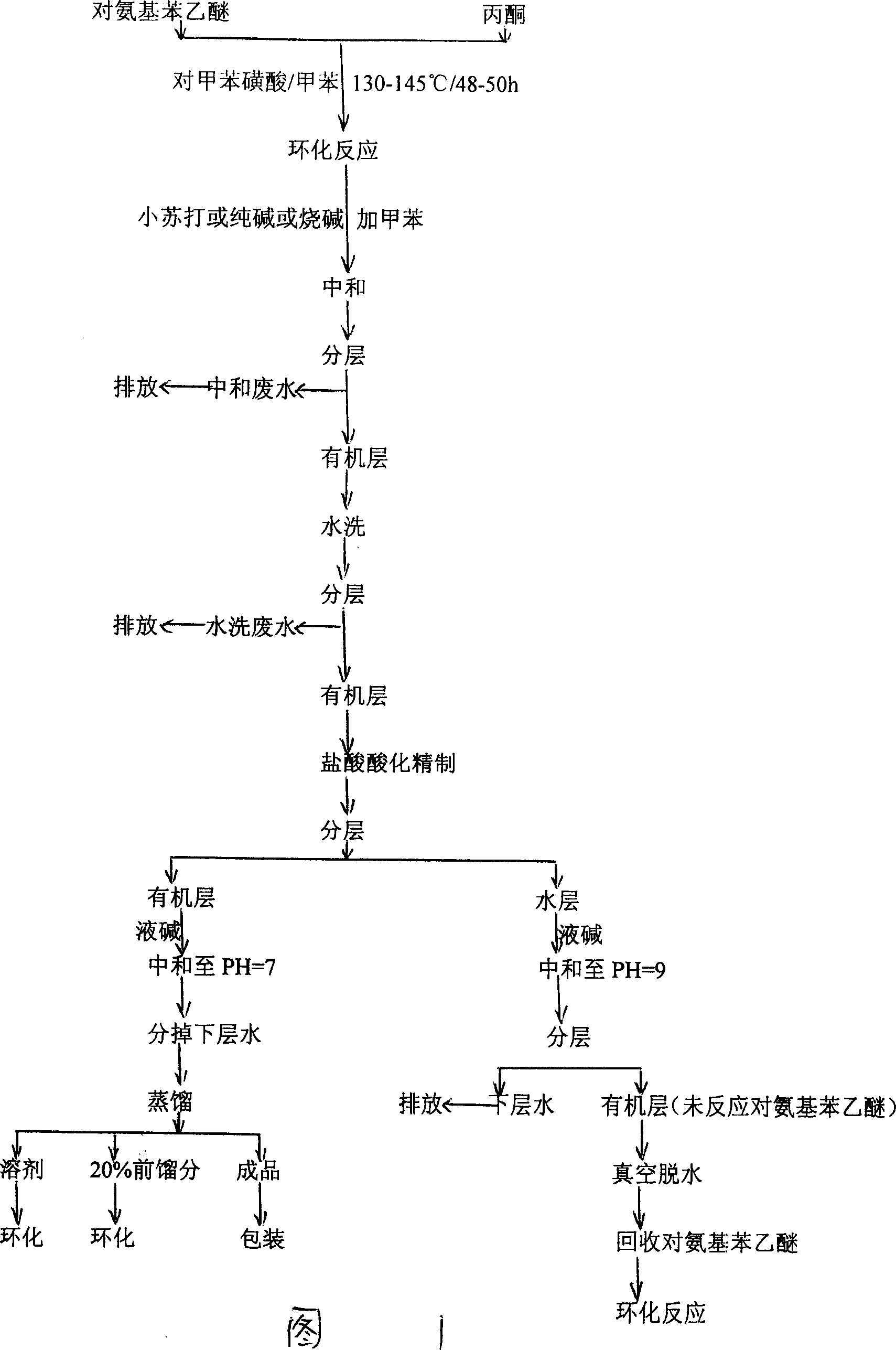
[0016] see figure 2 As shown in the process flow diagram, 1 mole of p-aminophenethyl ether is mixed with 2.07 moles of acetone material and placed in a vertical fixed-bed reactor, which is filled with 70% of the reactor volume H 2 SO 4 -MoO 3 -TiO 2 Solid superacid catalyst, at a temperature of about 150°C and a pressure of about 0.1Mpa, the cyclization reaction is about 30 hours, and the product after t...
Embodiment 2
[0018] The manufacture method of catalyst is with embodiment 1.
[0019] 1 mole of p-aminophenethyl ether is placed in a vertical fixed bed reactor with 2.1 moles of acetone, and the reactor is filled with 78% volume of H 2 SO 4 -MoO 3 -TiO 2 Solid superacid catalyst, at a temperature of about 160°C and a pressure of about 0.08Mpa, the cyclization reaction is about 35 hours, and the product after the cyclization reaction can be filtered and distilled to obtain the finished product, of which 10% of the previous fraction is re-distilled to obtain The finished product, the re-distilled 5% front cut is circulated into the fixed bed reactor for cyclization reaction.
Embodiment 3
[0021] The manufacture method of catalyst is with embodiment 1.
[0022] 1 mole of p-aminophenethyl ether is mixed with 2.2 moles of acetone material and placed in a vertical fixed-bed reactor filled with 65% volume of H 2 SO 4 -MoO 3 -TiO 2 Solid superacid catalyst, at a temperature of about 120°C and a pressure of about 0.15Mpa, the cyclization reaction is about 25 hours, and the product after the cyclization reaction can be filtered and distilled to obtain the finished product, of which 10% of the previous fraction is re-distilled to obtain The finished product, the re-distilled 5% front cut is circulated into the fixed bed reactor for cyclization reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com