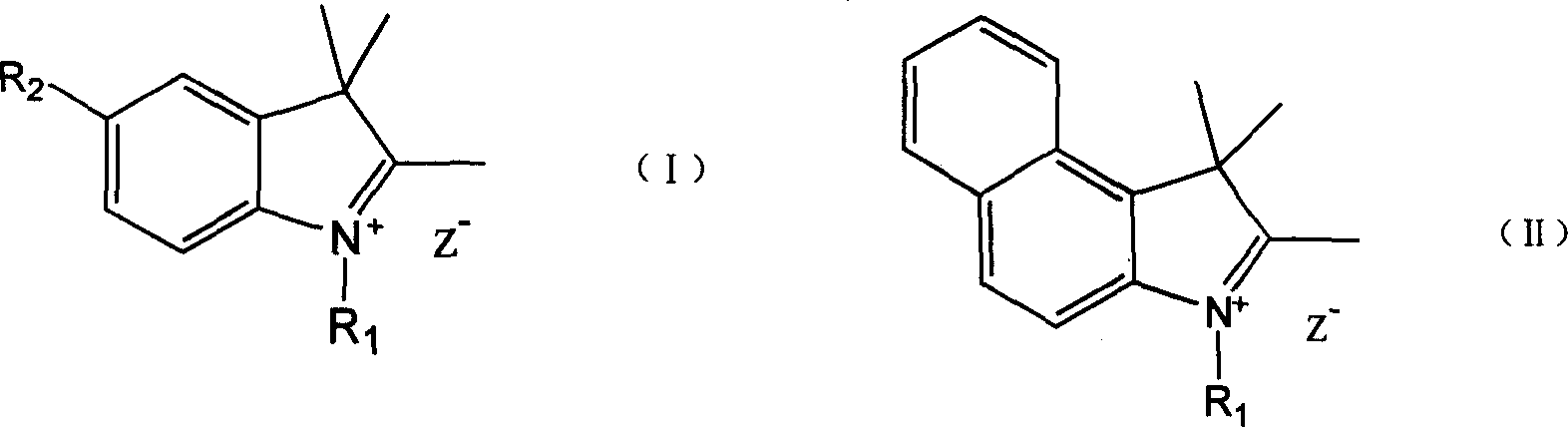
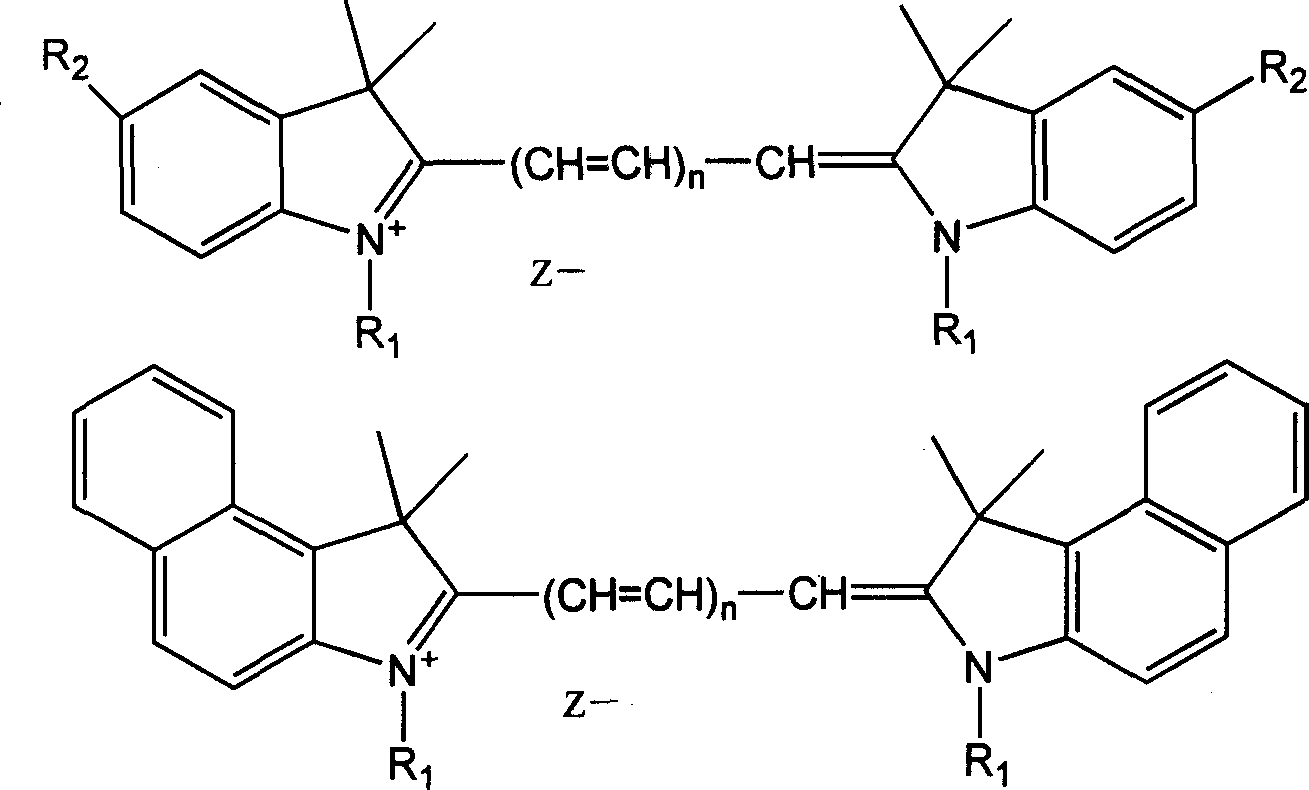
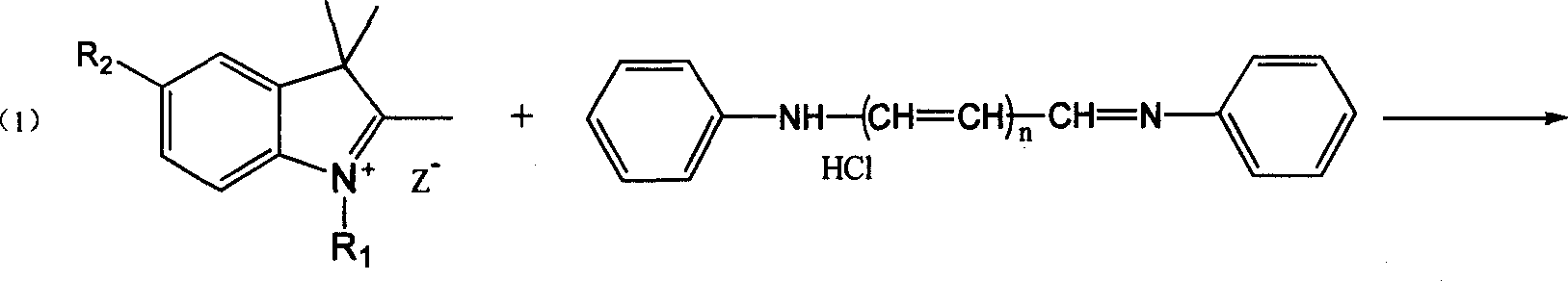
Process for synthesizing polymethin cyanine compound containing indole ring
The technology of a cyanine compound and a synthesis method, which is applied in the field of preparation of functional dyes, can solve the problems of difficult recycling, increased production costs, and easy hydrolysis of acetic anhydride, and achieves low production costs, avoids irritation, and has stable chemical properties Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 15ml of DMF or DMSO, 2ml of pyridine, 2.0g of 5-anilinopentadienal aniline hydrochloride, N-n-butyl-2,3,3-trimethylindoline perchlorate into a 100ml four-necked bottle Salt 5.0g, heated to 70°C with stirring, reacted for 3h, diluted with water, cooled to 20°C, filtered, washed with water, recrystallized from methanol, dried at 80°C to obtain heptamethine compound 1,1' containing indole ring -Di-n-butyl-3,3,3',3'-tetramethylindole tricarbocyanine perchlorate 3.0g.
Embodiment 2
[0026] Add 15ml of DMF, 4g of sodium acetate, 2.0g of 5-anilinopentadienal aniline hydrochloride, and N-n-butyl-2,3,3-trimethylindoline perchlorate into a 100ml four-necked bottle 5.0g, heated to 90°C with stirring, reacted for 2h, diluted with water, cooled to 20°C, filtered, washed with water, recrystallized from methanol, dried at 80°C to obtain heptamethine compound 1,1'-di n-Butyl-3,3,3',3'-tetramethylindole tricarbocyanine perchlorate 3.1g.
Embodiment 3
[0028] Add 15ml of DMF, 2.5ml of piperidine, 2.0g of 3-anilinopropenal aniline hydrochloride, N-n-butyl-2,3,3-trimethyl-4,5-benzoindole into a 100ml four-necked bottle Indoline perchlorate 6.0g, heated to 80°C with stirring, reacted for 2h, diluted with water, cooled to 15°C, filtered, washed with water, recrystallized with ethanol, dried at 80°C to obtain pentamethylcyanine compounds containing indole rings 1,1'-Di-n-butyl-3,3,3',3'-tetramethyl-4,5-benzoindole dicarbocyanine perchlorate 3.8g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com