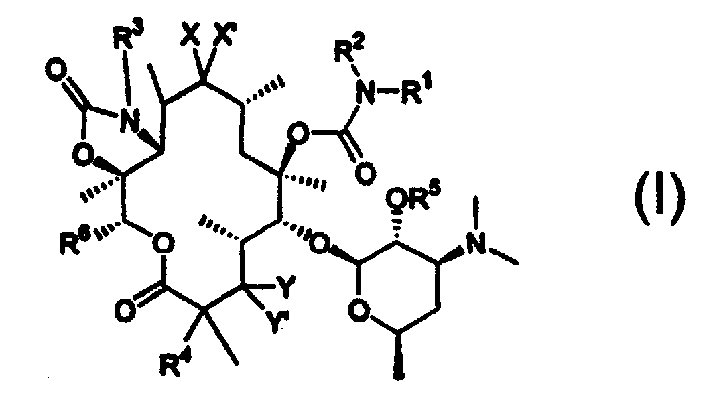
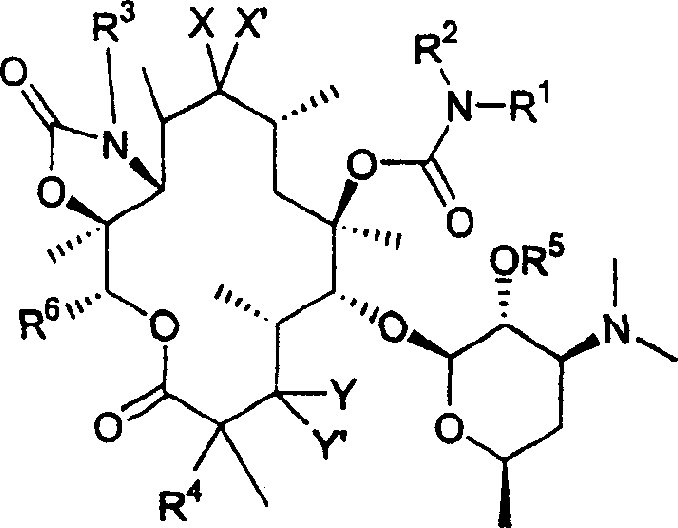
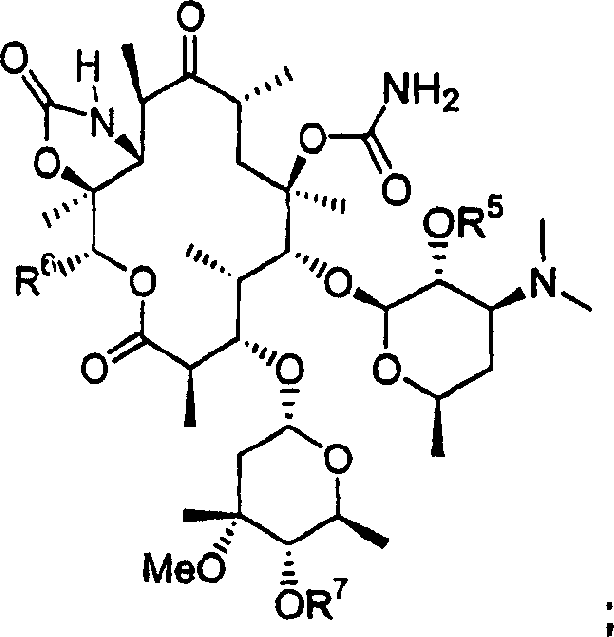
6-o-carbamoyl ketolide antibacterials
An alkyl and alkynyl technology, applied in the field of macrolide compounds, can solve the problems of low stability, poor absorption, instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0072]
[0073] Formula I'
[0074] where R 1 , R 2 , R 3 and R 4 as above. More specifically R 2 and R 3 is hydrogen and R 4 For fluorine.
[0075] The compound of formula 1 shown in formula 1 " further constitutes an embodiment of the present invention:
[0076]
[0077] Formula I"
[0078] where R 1 , R 2 , R 3 and R 4 as above. More specifically R 2 and R 3 are all hydrogen, and R 4 For fluorine.
[0079] where R 5 Compounds of formula 1 selected from acyl and aroyl form further embodiments of the invention.
[0080] The invention also provides methods of preparing the compounds of the invention.
[0081] Compounds of formula I can be prepared using readily available starting materials such as erythromycin and erythromycin derivatives known in the art. Schemes 1-18 describe representative methods for preparing inventive compounds:
[0082] plan 1
[0083]
[0084] Scheme 1 illustrates the precurso...
Embodiment 1
[0170] Embodiment 1. compound 6 (formula 1': R 1 for H, R 2 for H, R 3 for H, R 4 for H)
[0171]
[0172] Formula 1'
[0173] Step A
[0174] Triethylamine (42.0 mL, 301 mmol), DMAP (0.6 g, 4.9 mmol) and acetic anhydride (28.5 mL, 302 mmol). The temperature of the mixture was allowed to return to room temperature naturally, and stirred for 18 hours. Methanol (10 mL) was added and stirring was continued for 5 minutes. The mixture was diluted with ether (750 mL), followed by saturated NaHCO 3 Washed with aqueous solution, water and brine (500 mL each), dried (MgSO 4 ), the title compound was obtained as a colorless foam after concentration. The product was used directly in the next step without further purification. MS 860(M+H) + .
[0175] Step B
[0176] To a solution of Step A compound (50.0 mmol) in THF (500 mL) at 0° C. was added sodium hexamethyldisilazide (1.0 M in THF, 60.0 mL, 60.00 mmol) over 25 minutes. After 2 h at 0°C, the mixture w...
Embodiment 2
[0187] Example 2. Compound 106 (Formula 1': R 1 for H, R 2 for H, R 3 for H, R 4 for F)
[0188] To a solution of the compound from Example 1, Step F (520 mg, 0.76 mmol) in DMF (8 mL) was added sodium hexamethyldisilazide (1.0 M in THF, 1.14 mL, 1.14 mmol). After 30 minutes at -60°C, add SELECTFLUOR TM (324 mg, 0.91 mmol). The resulting mixture was stirred at -60°C for 10 min, diluted with ethyl acetate, washed with water and brine, dried (MgSO 4 ),concentrate. The resulting material was placed in methanol for 24 hours, then concentrated. Chromatography (SiO 2 , 3-5% methanol / dichloromethane + 0.1% concentrated NH 4 OH) and subsequent secondary chromatography (SiO 2 , acetone) to afford 201 mg (40%) of the title compound. MS 660(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com