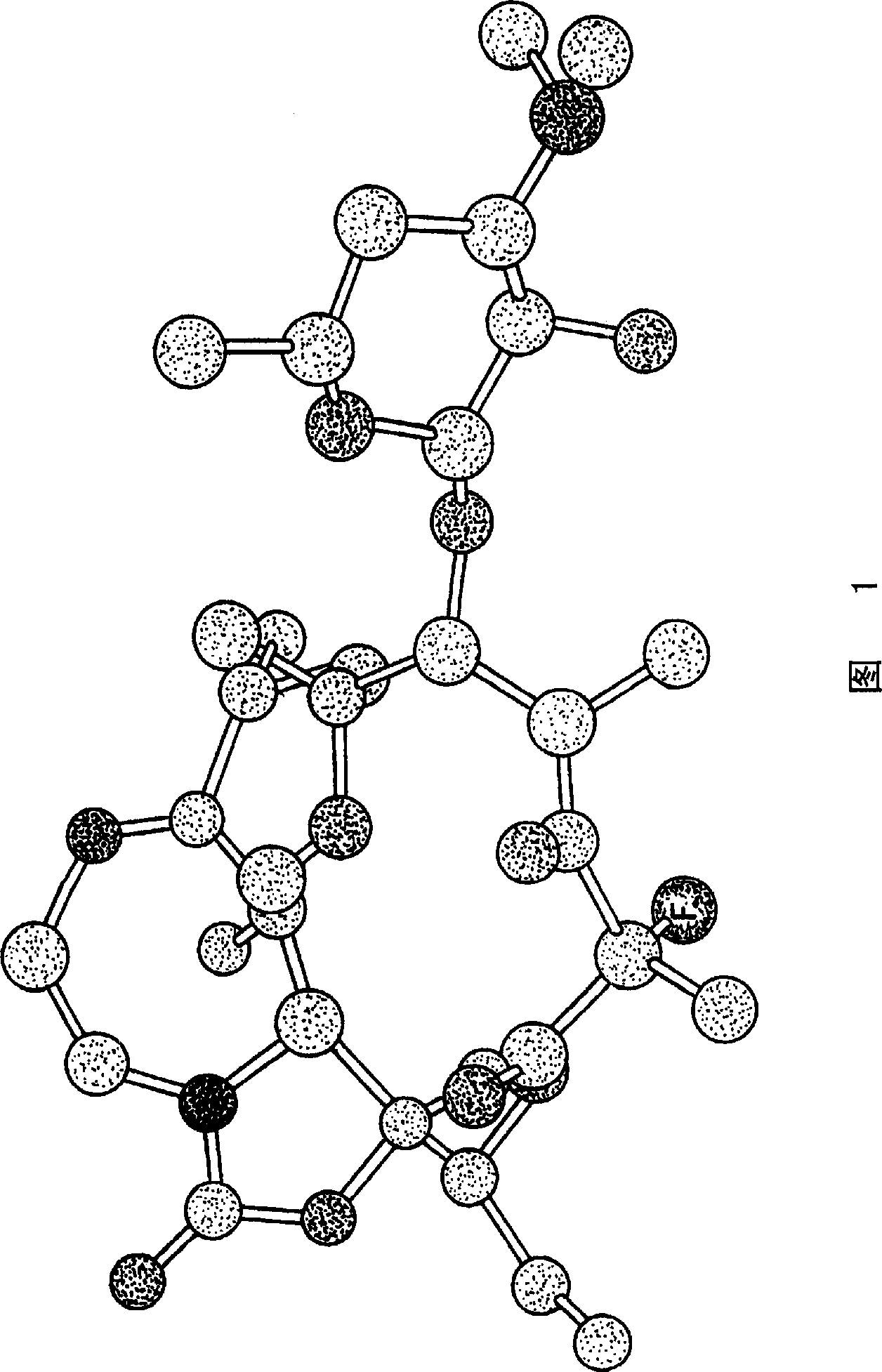
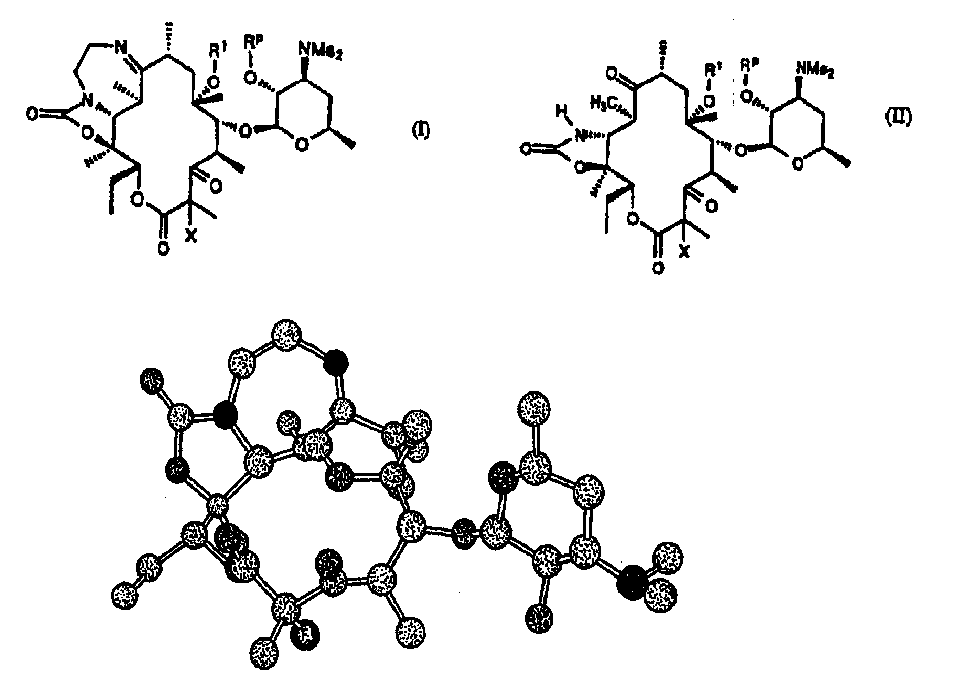
Z-halo-6-o-substituted ketolide derivatives
A technology of substituents and compounds, applied in the field of semi-synthetic macrolides, can solve problems such as erythromycin resistance or insufficient sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0189] Compound of formula (I): R p is H, R 1 Be methyl, X is F step 1a formula (I) compound: R p is benzoyl, R 1 is methyl, X is F
[0190] Scheme 3 compound 12 at 0°C (wherein R p is benzoyl, R 1 NaH (60% in oil, 108mg, 2.70mmol) was added to a solution of NaH (60% in oil, 108mg, 2.70mmol), and the mixture was stirred for 30 minutes . N-Fluorobenzenesulfonylimide (510 mg, 1.62 mmol, Aldrich) was added to the solution, and the mixture was stirred at 0°C for 3 hours. The mixture was taken up in 2-propyl acetate and washed successively with aqueous sodium bicarbonate and brine, dried over sodium sulfate and concentrated in vacuo. Chromatography on silica gel (eluting with 2:1 hexane:acetone) gave the title compound (615 mg). Step 1b Compound of formula (I): R p is H, R 1 is methyl, X is F
[0191] A sample (600mg, 0.791mmol) of the compound obtained in step 1a (solution) in methanol (25ml) was heated at reflux for 24 hours to remove the 2'-benzoyl group. The me...
Embodiment 2
[0195] In scheme 3 compound 12 (wherein R p is H, R 1 is methyl) (130mg, 0.204mmol, prepared according to U.S. Patent 5,631,355) in N-methylpyrrolidone (1.5ml) was added hexachloroethane (50mg, 0.214mmol) and sodium carbonate (43mg, 0.408 mmol). The mixture was stirred at room temperature for 3 days, further hexachloroethane (50 mg) and sodium carbonate (50 mg) were added, and the mixture was stirred at 60°C for 24 hrs. The mixture was dissolved in 2-propanol and washed sequentially with 5% aqueous sodium bicarbonate and brine, dried over sodium sulfate, and concentrated in vacuo. Chromatography on silica gel (eluting with acetone) gave the title compound (67 mg).
[0196] MS m / z672(M+H) + ;
[0197] 13 C NMR (75MHz, CDCl 3 )δ201.3, 181.0.165.8, 155.9, 104.1, 81.4, 80.7, 79.3, 80.0, 74.2, 70.4, 69.6, 65.9, 60.5, 49.6, 48.4, 42.8, 42.3, 42.2, 40.2, 38.9, 36.2, 31.8 .28.2, 22.1, 21.1, 19.7, 18.7, 16.5, 13.0, 11.0, 10.5;
[0198] HRMS m / z(M+H) + Analysis C 33 h 55 ClN...
Embodiment 3
[0200] Scheme 3 compound 12 at 0°C (wherein R p is H, R 1 is methyl) (500mg, 0.785mmol, prepared according to US Pat. 3 (352 mg, 1.10 mmol). The mixture was stirred at room temperature overnight, and pyridine·HBr was added 3 (2 equivalents), and the mixture was stirred at room temperature for 5 hours. The mixture was quenched with aqueous sodium carbonate and saturated aqueous sodium thiosulfate, pH 10. The mixture was extracted with dichloromethane, then washed successively with aqueous sodium carbonate and brine, dried over sodium sulfate, and concentrated in vacuo. Silica gel chromatography (with 3-5% (2M NH 3 methanol) and dichloromethane) to give the title compound (226 mg). MS (DCI / NH 3 )m / z 716( 79 Br(M+H) + ) and 718 ( 81 Br(M+H) + ); 13 C NMR (75 MHz, CDCl 3 )δ 201.6, 181.1, 166.1, 155.8, 103.6, 81.4, 80.1, 79.8, 78.8, 70.3, 69.4, 66.0, 65.8, 60.3, 49.4, 48.5, 44.0, 42.8, 42.2, 40.2, 38.8, 36.0, 32.5, 28.2, 20.0, 21.1, 19.6, 18.8, 17.4, 14.0, 13.0, 10.9,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com