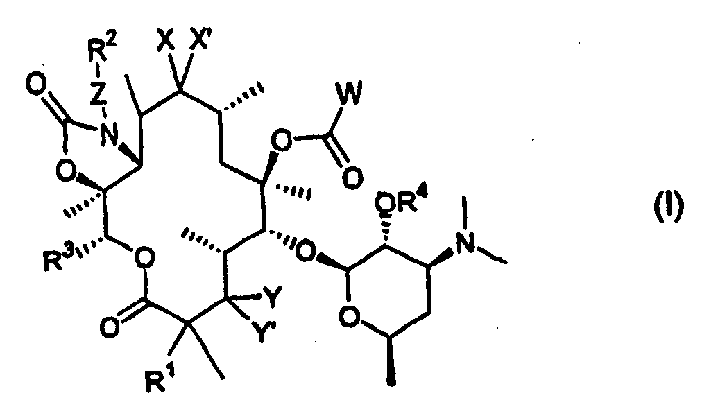
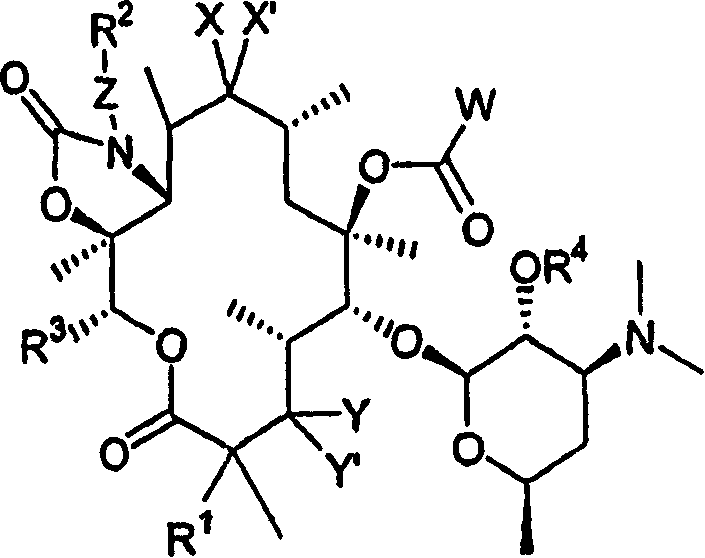
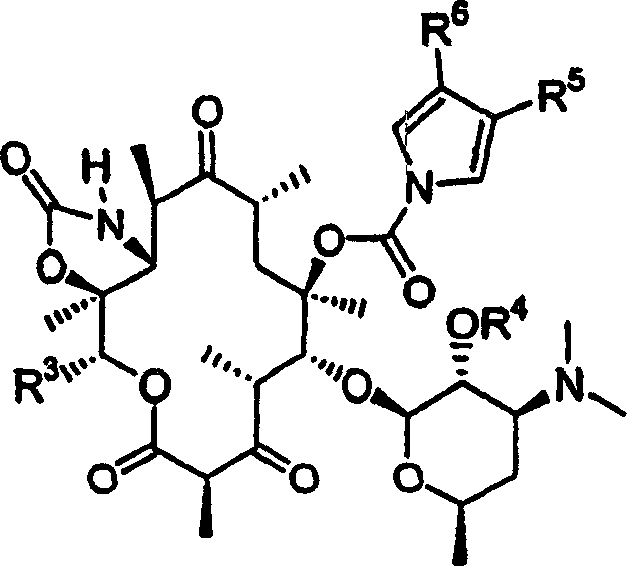
6-O-acyl ketolide derivatives of erythromycine useful as antibacterials
An alkynyl and alkyl technology, applied in the field of macrolide compounds, can solve the problems of low stability and unstable oral absorption of erythromycin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0212] Compound IX
[0213] Step A
[0214] Triethylamine (42.0 mL, 301 mmol), DMAP (0.6 g, 4.9 mmol) and acetic anhydride (28.5 mL, 302 mmol) were added to suspend erythromycin (36.7 g, 50 mmol) in dichloromethane (250 mL) at 0° C. in the liquid. The mixture was allowed to warm to room temperature and stirred for 18 hours. Methanol (10 mL) was added and stirring was continued for 5 minutes. The mixture was diluted with diethyl ether (750 mL), washed with saturated aqueous sodium bicarbonate, water and brine (500 mL each), dried (magnesium sulfate) and concentrated to afford the title compound as a colorless foam. The material was used directly in the next step without further purification. MS860(M+H) + .
[0215] Step B
[0216] Sodium hexamethyldisilylamide (1.0 M in tetrahydrofuran, 60.0 mL, 60.00 mmol) was added to a solution of the compound from Step A (50.0 mmol) in THF (500 mL) at 0° C. over 25 minutes. After 2 hours at 0 °C, the mixture was diluted with water (...
Embodiment 2
[0228] Compound 2 (formula 1a: R 5 for H, R 6 for H)
[0229] A solution of compound IX (1.00 g, 1.56 mmol), 2,5-dimethoxytetrahydrofuran (0.40 mL, 3.09 mmol) and trifluoroacetic acid (0.60 mL, 7.79 mmol) in acetonitrile (10 mL) was stirred at room temperature for 24 hours . Water (5 mL) was added and the solution was stirred for 20 hours. The reaction mixture was diluted with ethyl acetate (75 mL), washed with saturated aqueous sodium bicarbonate (50 mL) and brine (50 mL), dried over magnesium sulfate and concentrated. Purified by chromatography (SiO 2 , 95:5:0.2 dichloromethane / methanol / conc. ammonia) afforded 550 mg (51%) of the title compound. MS 692(M+H) + .
Embodiment 3
[0231] Compound 3 (formula 1a: R 5 is C(O)H, R 6 for H)
[0232] A solution of compound IX (500 mg, 0.78 mmol), 2,5-dimethoxy-3-tetrahydrofurfuraldehyde (625 mg, 3.90 mmol) and trifluoroacetic acid (0.60 mL, 7.79 mmol) in acetonitrile (5 mL) was stirred at room temperature 18 hours. The reaction mixture was diluted with ethyl acetate (50 mL), washed with saturated aqueous sodium bicarbonate (50 mL) and brine (50 mL), dried over sodium carbonate and concentrated. Purified by chromatography (SiO 2 , 95:5:0.5 dichloromethane / methanol / conc. ammonia) afforded 255 mg (45%) of the title compound. MS 720(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com