Electrolyte composition for electrolysis of brine, method for electrolysis of brine, and sodium hydroxide prepared therefrom
A salt water electrolysis and electrolyte technology, applied in electrolysis components, electrolysis process, electrochemical water/sewage treatment, etc., can solve problems such as reduced productivity, unusable electrolysis cells, consumption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
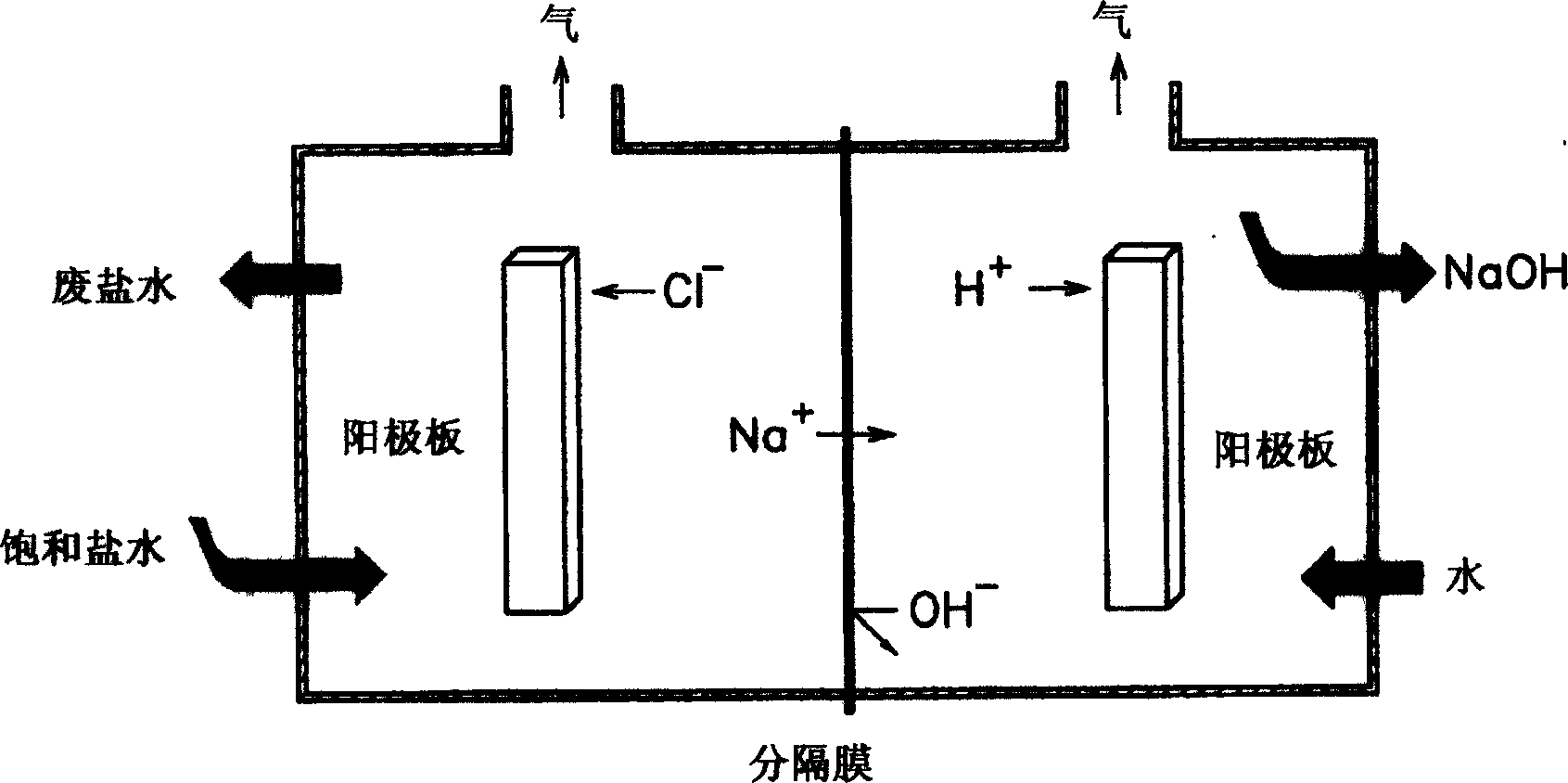
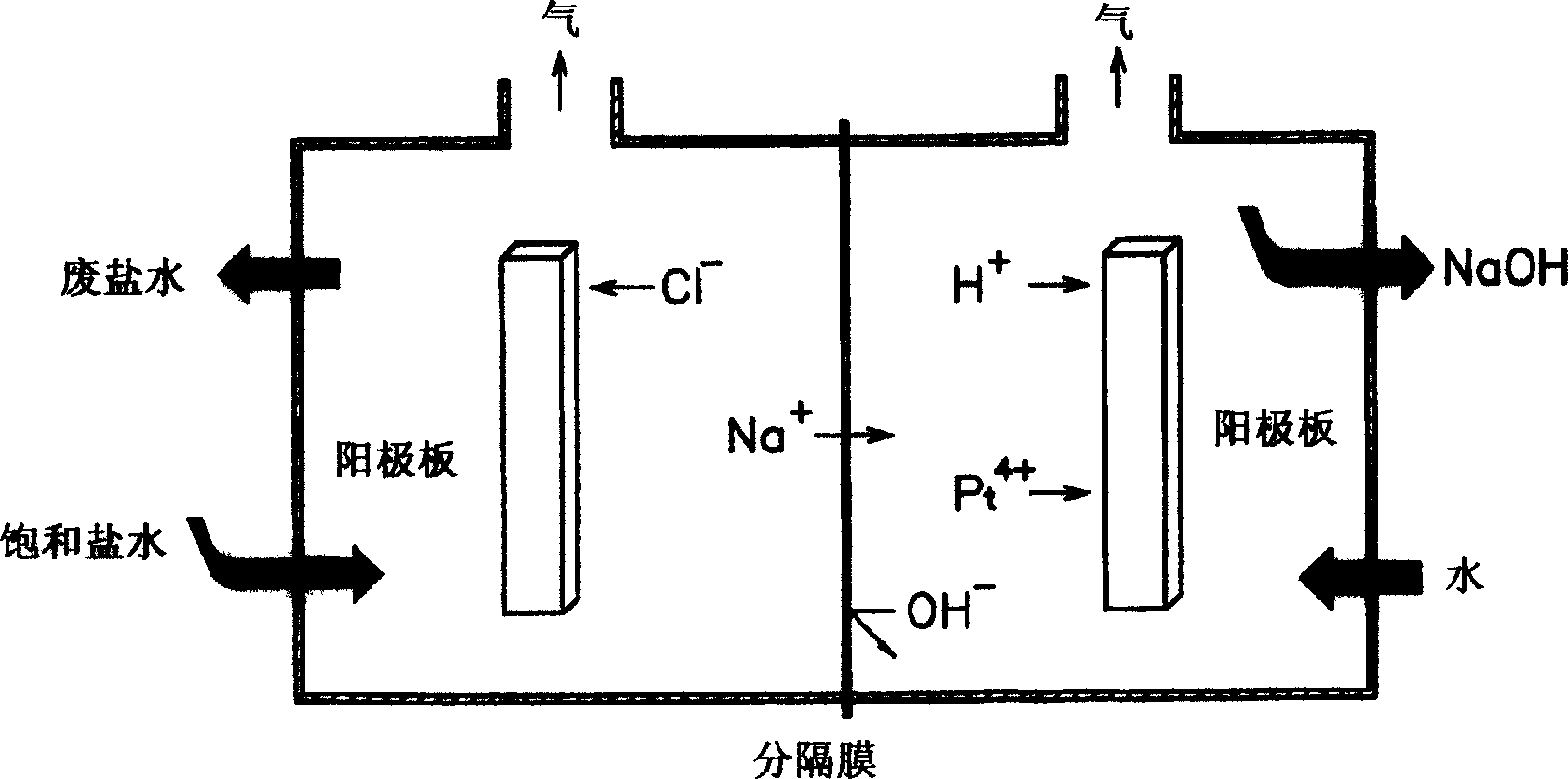
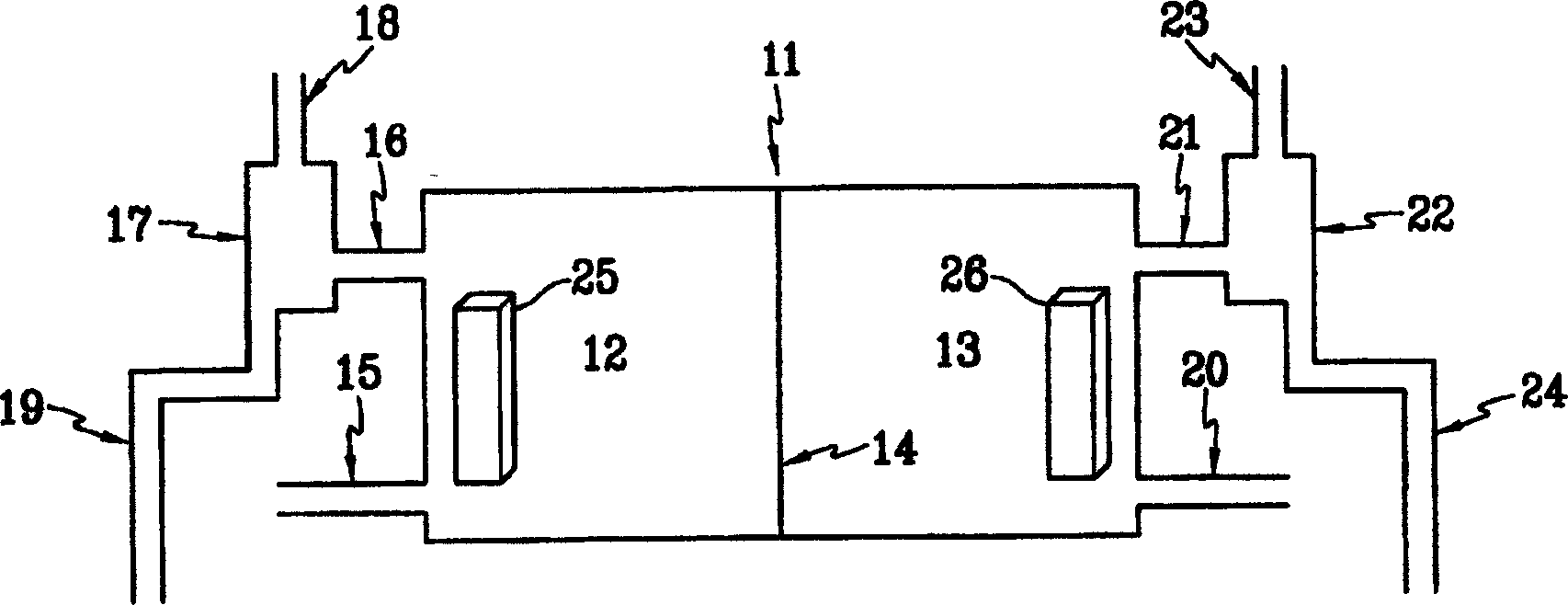
[0071] In 1 liter of pure water, add 10 g of hexachloroplatinate (IV) (H 2 PtCl 6 ·6H 2 O) to prepare an aqueous solution of hexahydroxyhydroplatinate (IV). This aqueous solution and pure water were injected into the platinum compound aqueous solution injection pipe and the pure water injection pipe in the electrolytic cell, respectively. Brine was injected into the electrolytic cell, an electrolyte composition containing the prepared platinum compound aqueous solution was injected into a cathode circulation tube, and the brine was electrolyzed for 3 minutes to prepare an aqueous sodium hydroxide solution. The total amount of pure water injected was 10 liters, and the total amount of hexachloroplatinate (IV) injected was 1 liter.
Embodiment 2
[0073] An aqueous sodium hydroxide solution was prepared in the same manner as in Example 1, except that potassium tetrachloroplatinate (II) (K 2 PtCl 4 ) as a platinum compound.
Embodiment 3
[0075] Aqueous sodium hydroxide was prepared in the same manner as in Example 1, except that diaminodinitroplatinum (II) (Pt(NH) was used. 3 ) 2 (NO) 2 ) as a platinum compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com