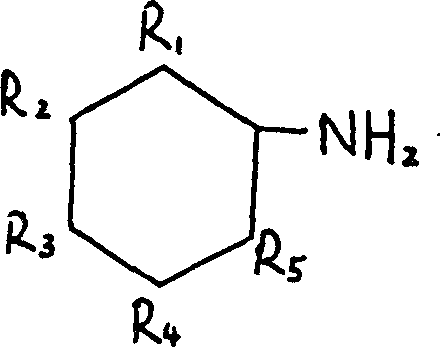
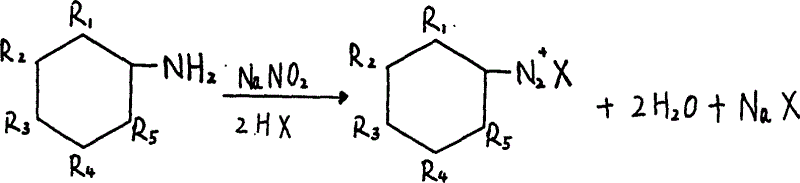
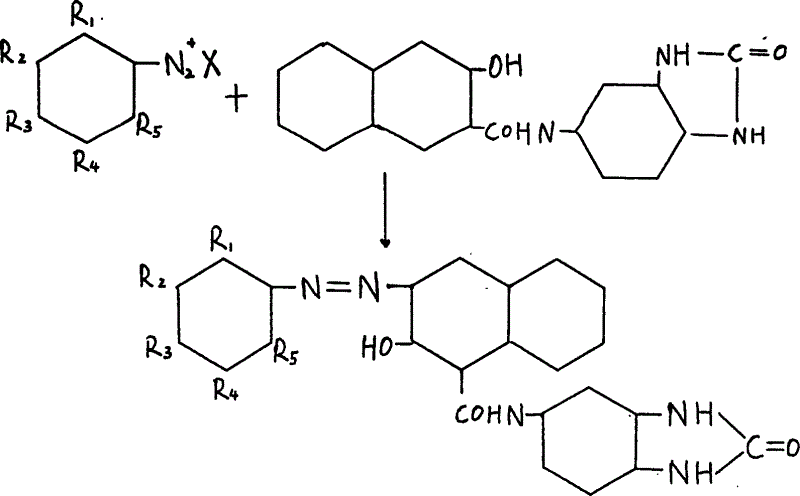
Process for preparing red benzimidazoleone pigments
A technology of benzimidazolone and pigment, applied in the field of fine chemical industry, can solve the problem of too many organic solvents, and achieve the effect of high tinting strength, excellent performance and bright color and light.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of 171# red benzimidazolone pigment
[0044] (1) Add 7.4g of 4-nitro-o-methoxyaniline and 0.2g of methyl anthranilate (the ratio of the two components is 37:1) into 240 ml of deionized water, stir for 30 minutes, add 10% polyoxygen Vinyl ether derivative solution 3.8ml, 30% hydrochloric acid 20ml, cooled to below 0°C in an ice-salt bath, slowly added 18.5ml of sodium nitrite solution (20%), with a slight excess of sodium nitrite at the end point, stirred for 10 minutes, added Activated carbon, stirred and adsorbed, filtered, and the filtrate was to be coupled.
[0045] (2) 15g of 5-(2'-hydroxyl-3'-naphthoyl)aminobenzimidazolone was added to 190 milliliters of deionized water, 30% sodium hydroxide 15ml, 10% castor oil sodium sulfonate 3.8ml, stirred Until it is completely dissolved, add a small amount of activated carbon to filter, add ion-free ice water to the filtrate to a volume of 550 ml, and use 5% glacial acetic acid solution to precipitate fine partic...
Embodiment 2
[0050] Preparation of 175# red benzimidazolone pigment
[0051](1) Add 8.0g of methyl anthranilate and 0.16g of 3-amino-4-methoxybenzanilide (the ratio of the two components is 50:1) into 240 ml of deionized water, stir for 30 minutes, add 10 3.8ml of % polyoxyethylene ether derivative solution, 23ml of 30% hydrochloric acid, cooled to below 0°C in an ice-salt bath, slowly added 18.5ml of sodium nitrite solution (20%), with a slight excess of sodium nitrite at the end point, stirred for 10 Minutes, add activated carbon, stir and adsorb for 10 minutes, filter, and the filtrate is to be coupled.
[0052] (2) 15 g of 5-(2'-hydroxy-3'-naphthoyl)aminobenzimidazolone was added to 190 ml of deionized water, 15 ml of 30% sodium hydroxide, 2 ml of 10% sodium castor oil sulfonate liquid, and stirred Until it is completely dissolved, add a small amount of activated carbon to filter, add ion-free ice water to the filtrate to a volume of 550 ml, and use 10% glacial acetic acid solution to...
Embodiment 3
[0057] Preparation of 175# red benzimidazolone pigment
[0058] (1) Add 12.5 g of 3-amino-4-methoxybenzanilide and 0.125 g of methyl anthranilate (the ratio of the two components is 100:1) into 240 ml of deionized water, stir for 30 minutes, add 10% polyoxyethylene ether derivative solution 3.8ml, 30% hydrochloric acid 20ml, cool to below 0°C in an ice-salt bath, slowly add 18.5ml of sodium nitrite solution (20%), the end point is a slight excess of sodium nitrite, stir After 10 minutes, activated carbon was added, stirred and adsorbed for 10 minutes, filtered, and the filtrate was to be coupled.
[0059] (2) 15 g of 5-(2'-hydroxyl-3'-naphthoyl)aminobenzimidazolone was added to 190 ml of deionized water, 30% sodium hydroxide 15 ml, 10% castor oil sodium sulfonate solution 3.8 ml, Stir until completely dissolved, add a small amount of activated carbon and filter.
[0060] Add the diazonium solution to the coupling solution dropwise in 20-50 minutes, the coupling solution is s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com