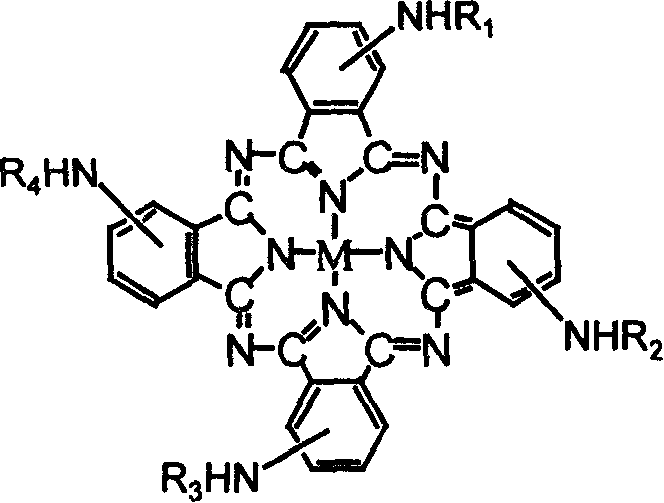
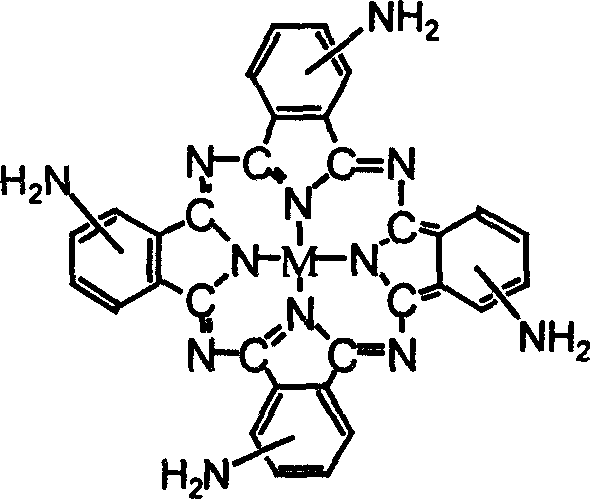
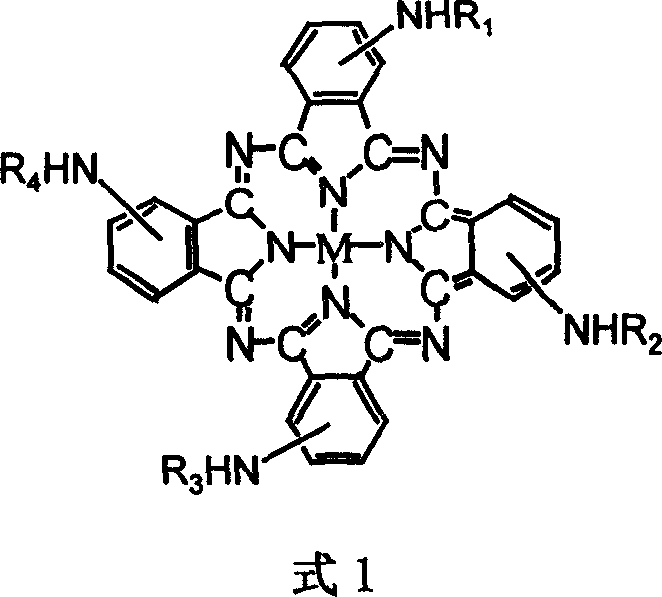
Metalphthalocyanine derivative, salt and preparing method thereof
A technology of metal phthalocyanine and derivatives, applied in the field of preparation of new metal phthalocyanine derivatives and salts, can solve the problems of poor product purity, complicated separation, harsh reaction conditions, etc., and achieve good water solubility, high yield and purity , The effect of simple preparation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Dissolve 3.0g tetraaminocobalt phthalocyanine in 100mL DMF, and dissolve 1.76g maleic anhydride in 20mL DMF solution, and slowly add it dropwise to tetraaminocobalt phthalocyanine solution, react at 60°C for 3h, pour the reaction solution Pour into 500mL water and let stand overnight. After the phthalocyanine is completely precipitated, it is centrifuged and dried to obtain a crude tetramaleic acid amide cobalt phthalocyanine product.
Embodiment 2
[0022] Dissolve 3.0g tetraaminocobalt phthalocyanine in 100mL DMF, and dissolve 1.90g maleic anhydride in 20mL DMF solution, and slowly add it dropwise to the tetraaminocobalt phthalocyanine solution, react at 60°C for 3 hours, pour the reaction solution Pour into 500mL water and let stand overnight. After the phthalocyanine was completely precipitated, it was separated by centrifugation and dried. The product was redissolved in 50ml of 0.01mol / L NaOH solution, filtered to remove insoluble matter, and the filtrate was adjusted to pH 2 with 1.0mol / L HCl solution, the product was precipitated and separated by centrifugation, and this process was repeated 2 times and dried in a vacuum oven to obtain tetramaleic acid amide cobalt phthalocyanine.
Embodiment 3
[0024] Dissolve 3.0g tetraaminocobalt phthalocyanine in 100mL DMF, and dissolve 1.90g succinic anhydride in 20mL DMF solution, and slowly add it dropwise into tetraaminocobalt phthalocyanine solution, react at 60°C for 3h, pour the reaction solution Pour into 500mL water and let stand overnight. After the phthalocyanine was completely precipitated, it was separated by centrifugation and dried. The product was redissolved in 50ml of 0.01mol / L NaOH solution, filtered to remove insoluble matter, and the filtrate was adjusted to pH 2 with 1.0mol / L HCl solution, the product was precipitated and separated by centrifugation, and this process was repeated 2 times and dried in a vacuum oven to obtain tetrasuccinamide cobalt phthalocyanine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com