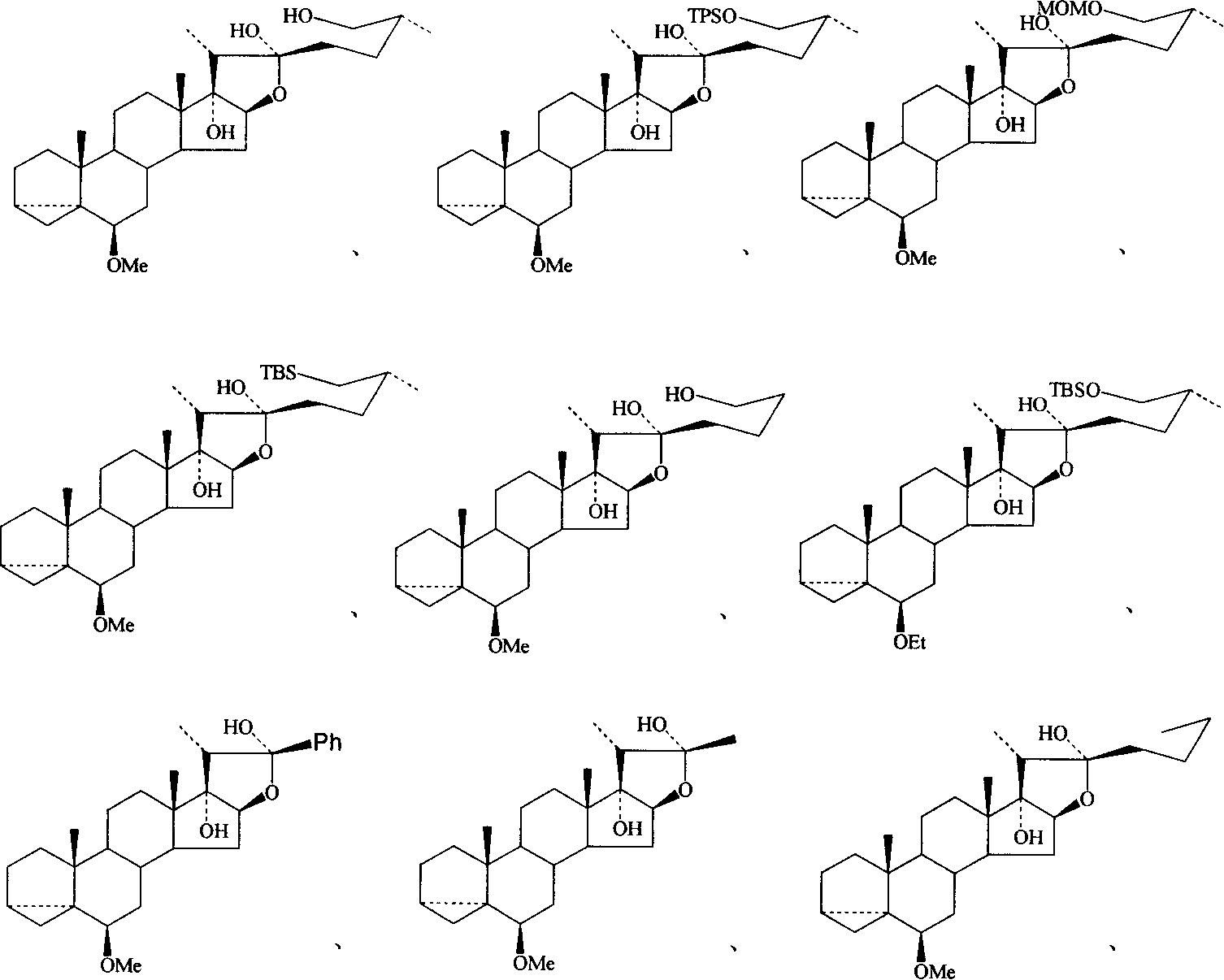
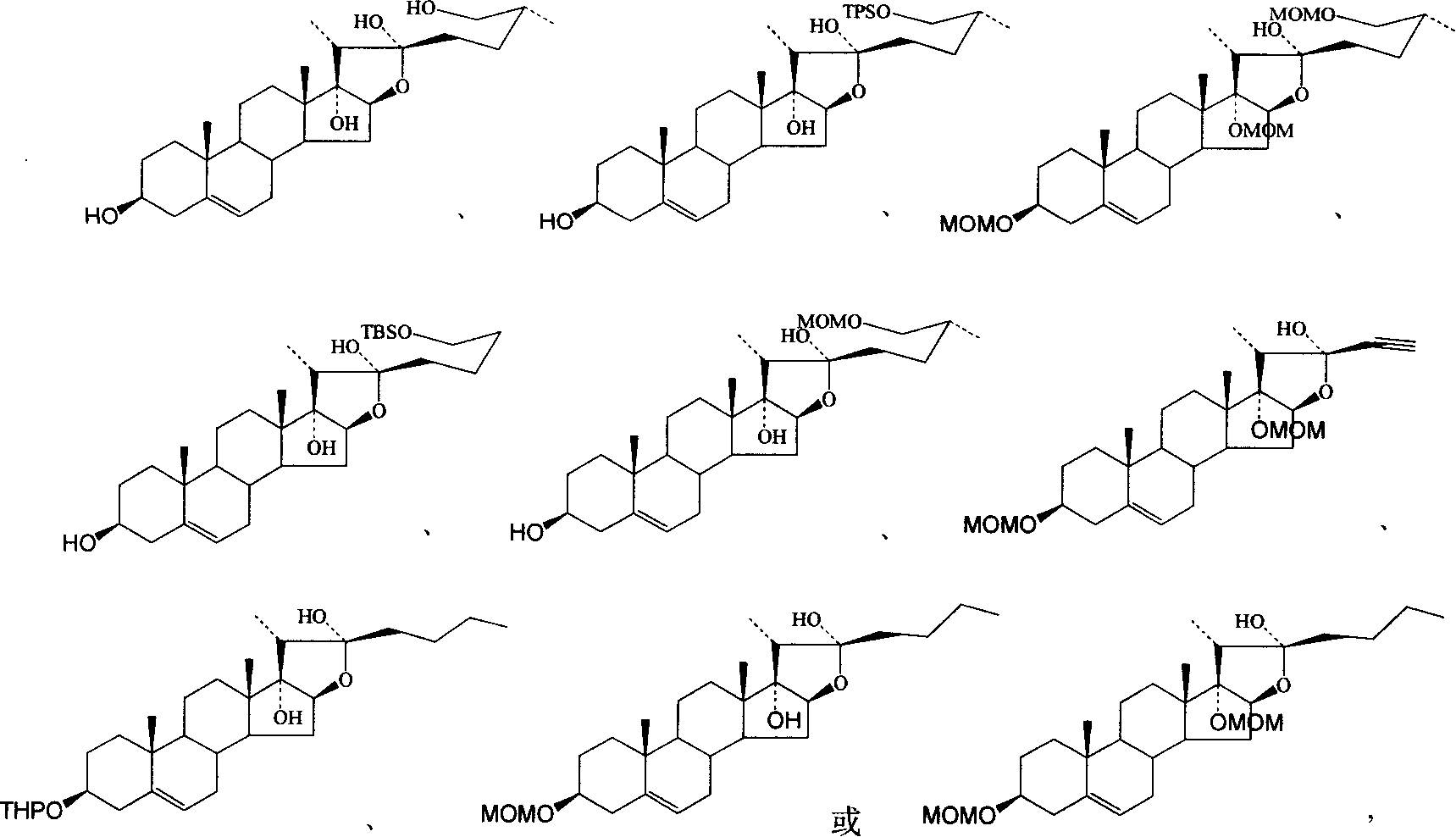
17 alpha, 22-dicarboxy furo compound and its use
A technology of hydroxyfurostate compound and diphenyl, applied in the field of 17α, can solve the problems of exacerbating the shortage of plant resources, affecting the ecological environment, destroying vegetation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The synthesis of embodiment 1 compound 2
[0028]
[0029] Preparation of 3α,5α-cyclo-6β-methoxy-17α,20R-furostediol:
[0030] Add 2-5 equivalents of lithium reagent (or Grignard reagent) dropwise into 1 equivalent of steroid lactone 1 in 5 mL of anhydrous THF solution, and prepare furostane compound 3α, 5α-cyclo-6β-methoxy at -20°C - 17α, 20R-Furostanediol 2 (20-90% yield).
[0031] Compound 2a (5 equivalents of lithium reagent, 64% yield)
[0032] 1 H-NMR (300MHz, CDCl 3 )δ: 7.66(m, 4H) and 7.36(m, 6H) (phenyls of TPS), 4.15(dd, 1H, J=4.8, 7.2Hz, H-16α), 3.48(m, 2H, H-26) , 3.32(s, 3H, MeO-), 2.78(dd, 't'like, J=2.4Hz, H-6α), 2.20(q, 1H, J=7.5Hz, H-20), 1.05(s, 9H, t-butyl of TPS), 1.04(s, 3H, H-19), 0.94(d, 3H, J=7.2Hz, H-21), 0.89(d, 3H, J=6.6Hz, H-27 ), 0.86 (s, 3H, H-18), 0.66 (dd, 't'like, J=4.0Hz, H-4β), .0.44 (dd, 1H, J=5.2, 7.9Hz, H-3α) .
[0033] ESIMS: m / z 701 (M + +1).
[0034] Compound 2b (3 equivalents of lithium reagent, 20% yield)
[0...
Embodiment 2
[0046] The synthesis of embodiment 2 compound 3
[0047]
[0048] Preparation of Δ5,6-3β,17α,20R-furosttriol:
[0049] Add 2-5 equivalents of lithium reagent (Grignard reagent) dropwise into 5 mL of anhydrous THF solution of 1 equivalent of steroidal lactone 1, and prepare furostanoid compound Δ5,6-3β,17α,20R-furostan at -20°C Triol 3 (50-89% yield). Compound 3a (R 1 =R 2 =H, ) (5 equivalents of lithium reagent, 50% yield)
[0050] 1 H-NMR (300MHz, CDCl 3 )δ: 7.66 (m, 4H) and 7.36 (m, 6H) (phenyls of TPS), 5.54 (d, 1H, J = 8.4Hz, H-6), 4.20 (dd, 1H, J = 4.8, 7.2Hz , H-16α), 3.48(m, 2H, H-26), 3.44(m, 1H, H-3β).2.24(q, 1H, J=7.5Hz, H-20), 1.05(s, 9H, t-butyl of TPS), 1.104 (s, 3H, H-19), 0.98 (d, 3H, J=7.2Hz, H-21), 0.83 (d, 3H, J=6.6Hz, H-27), 0.80(s, 3H, H-18),
[0051] ESIMS: m / z 673 (M + +1).
[0052] Compound 3b (R 1 = MOM,R 2 = H, R 4 =nBu) (3 equivalents of lithium reagent, 89% yield)
[0053] 1 H-NMR (300MHz, CDCl 3 )δ: 5.57(d,...
Embodiment 3
[0064] The synthesis of embodiment 3 compound 4
[0065]
[0066] (25R)-3α, 5α-cyclo-6β-methoxy-26-tert-butyldiphenylsilyloxy-17α, 20R-furostanediol desilication protecting group: 31mg (0.043mmol) furostanes Compound 2a was dissolved in 2 mL of anhydrous THF. Inject 0.43 mL (0.43 mmol) of 1M TBAF in THF, raise the temperature to ~60°C, and stir the reaction. Cool to room temperature, add 10 mL of water, extract with ethyl acetate, and wash the ester layer with saturated NaHCO 3 and NaCl solution, washed with anhydrous Na 2 SO 4 After drying, it was concentrated under reduced pressure. Add 10mgPPTS and 2mL THF to the obtained crude product, stir at room temperature for 2 hours, concentrate under reduced pressure, and obtain oily form by column chromatography: oily (25R)-3α,5α-cyclo-6β-methoxy-17α-spirosterol 19 17mg (81 %Yield).
[0067] Compound 4
[0068] 1 H-NMR (300MHz, CDCl 3 )δ: 3.93(dd, 't'like, 1H, J=7.6Hz, H-16α), 3.48(dd, 1H, J=3.9, 10.6Hz, H-26α), 3.37(dd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com