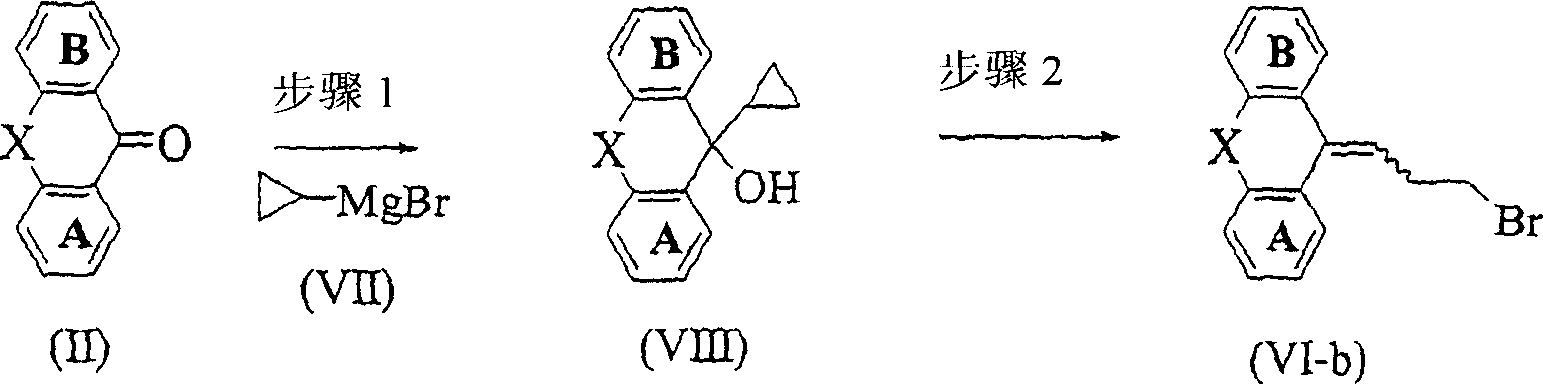Chemokine receptor antagonists and methods of use thereof
A compound and aliphatic-based technology, applied in anti-inflammatory agents, anti-viral agents, organic chemistry, etc., can solve problems such as unsuccessful development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
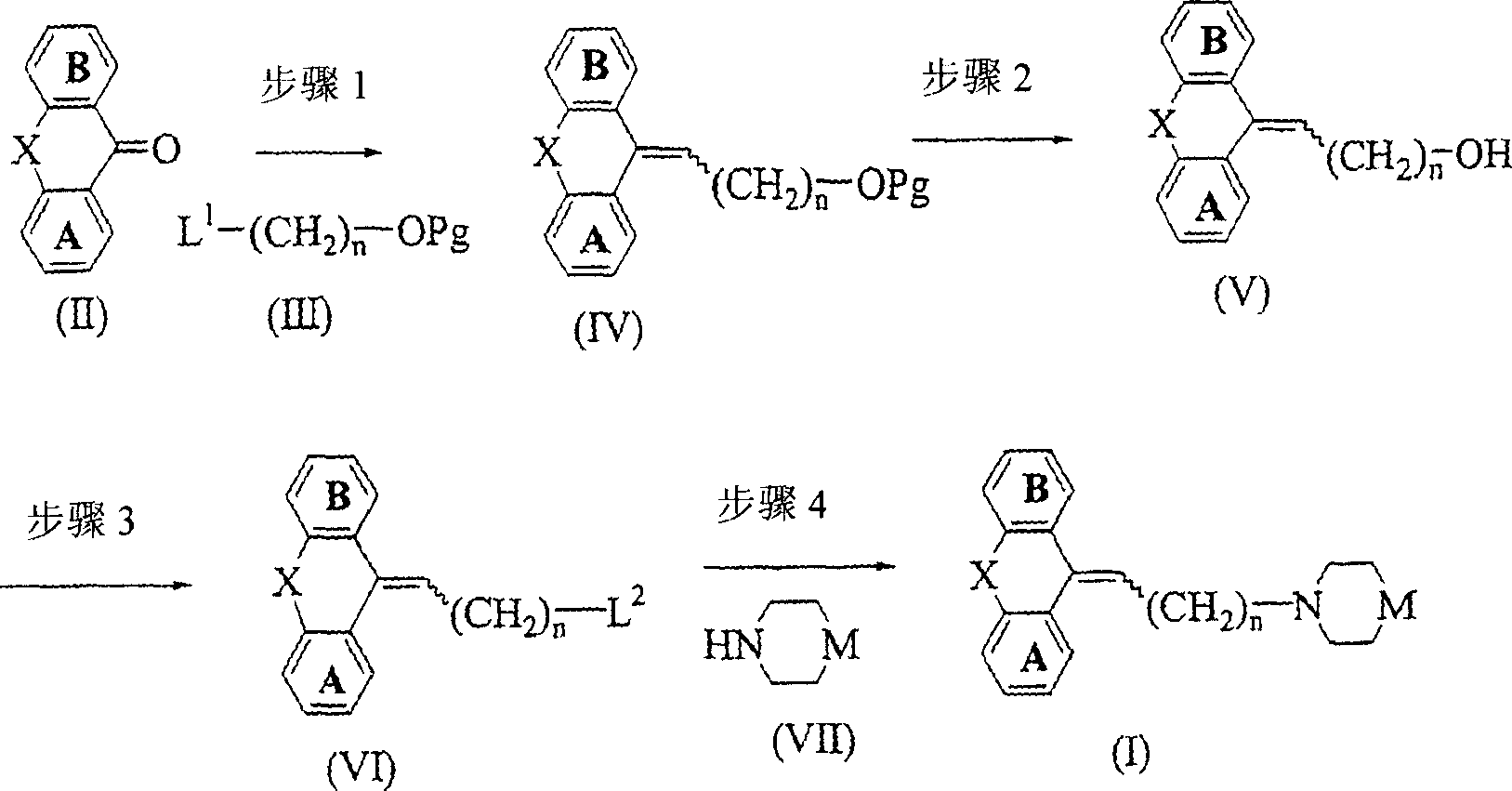
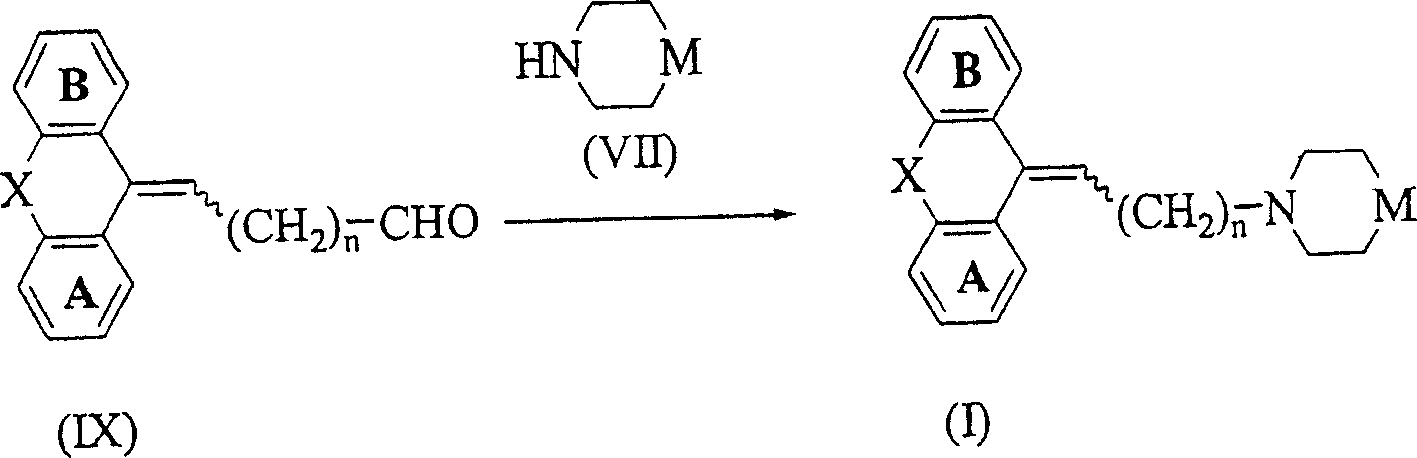
Image
Examples
Embodiment 1
[0234] Example 1: 4-(4-chlorophenyl)-1-[3-(10,11-dihydro-5H-
[0235] Dibenzo[a,d]cyclohepten-5-ylidene)propyl]piperidin-4-ol
[0236] To a solution of 5-(3-bromopropylene)-10,11-dihydro-5H-dibenzo[a,d]cycloheptene (described in JP48-030064) (200mg) in DMF (10ml) 4-(4-Chlorophenyl)-4-hydroxypiperidine (230 mg), potassium carbonate (360 mg) and potassium iodide (50 mg) were added. The mixture was stirred at 70°C for 24 hours. Ethyl acetate was added to the reaction mixture, and the organic layer was separated, washed with saturated aqueous sodium chloride solution, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure. The residue was purified by silica gel chromatography eluting with ethyl acetate-hexane (1:1) to give the title compound (250 mg). 1 H-NMR (CDCl 3 )d: 1.65-2.11(5H, m), 2.32-3.10(8H, m), 3.22-6.67(4H, m), 5.87(1H, t), 7.03-7.44(12H, m).MS m / z : 444(M+1).
Embodiment 2
[0237] Example 2: 4-(4-chlorophenyl)-1-[3-(6,11-dihydrodibenzo[b,e]
[0238] Oxaheptin-11-ylidene)propyl]piperidin-4-ol
[0239]The title compound was prepared following the procedure of Example 1, except that 11-(3-bromopropylene)-6,11-dihydrodibenzo[b,e]oxepin was used instead of 5-(3-bromopropylene base)-10,11-dihydro-5H-dibenzo[a,d]cycloheptene. 1 H-NMR (CDCl 3 )d: 1.61-2.16 (5H, m), 2.37-2.80 (8H, m), 5.22 (2H, brs), 5.70 (0.6x1H, t), 6.03 (0.4x1H, t), 6.73-6.90 (2H, m), 7.09-.7.45 (10H, m). MS m / z: 446 (M+1).
Embodiment 3
[0240] Example 3: Membrane preparation and binding detection of chemokine binding
[0241] Membranes were prepared using THP-1 cells (ATCC #TIB202). Cells were collected by centrifugation and washed twice with PBS (phosphate buffered saline). Cell pellets were frozen at -70 to -85°C. The frozen pellet was placed in an ice-cooled medium containing 5mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethane-sulfonic acid) pH 7.5, 2mM EDTA (ethylenediaminetetraacetic acid), each 5g / ml Aprotinin, leupeptin and chymostatin (protease inhibitors), and 100g / ml PMSF (phenylmethylsulfonyl fluoride - also a protease inhibitor) were dissolved in lysis buffer at a concentration of 1 to 5×10 7 cells / ml. This step lyses the cells. Mix the suspension well and resuspend any frozen cell pellets. Centrifuge at 400xg for 10 minutes at 4°C to remove nuclei and cell debris. Transfer the supernatant to a clean test tube and centrifuge at 25,000xg for 30 minutes at 4°C to collect membrane fragments. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com