Therapeutically useful triethyleneglycol cholesteryl oligonucleotides
A technology of cholestyl group and purpose, applied in the field of cholestyl group-coupled oligonucleotide composition, can solve the problems of toxicity, receptor weakening, immune sensitization and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Nucleotide sequence preparation
[0071] Phosphodiester synthetic nucleotide sequences and coupled 3'-TEG cholestenyl synthetic nucleotide sequences were prepared by Sigma-Genosys (Woodlands, TX, USA) using Abacus segment synthesis technology. A sequence with a TEG cholestenyl group at its 3' end was synthesized on a TEG CPG support (Glen Research, Sterling, VA, USA). The sequence was dispersed in water or in dimethyl sulfoxide (DMSO) immediately before use.
Embodiment 2
[0073] cell
[0074] All cell lines were obtained from the American Type Culture Collection (ATCC, Rockyille, MD) and were grown in media recommended by ATCC. Breast cancer cell lines were cultured in media recommended by ATCC or described in published literature (Herrera-Gayol and Jothy, Int. J. Exp. Path., 82: 193, 2001). Table 1 shows the cell lines described in relevant literature, the source of the cell lines and their biopathological characteristics (Hackett et al., Cancer Inst., 58: 1795, 1977; Price et al., Cancer Res., 50: 717, 1990; Thompson et al., J. Cell Physiol., 150:534, 1992; and Peiper M, et al., Int. J. Cancer 71:993, 1997).
[0075] Table 1
[0076] cell line
illustrate
MEG-01*
Human chronic myeloid leukemia cell line
EL-4*
HIG-82**
Rabbit synovial cell line
MCF-7**
Human, breast adenocarcinoma (pleural effusion). Well differentiated. in vitro
and non-invasive ...
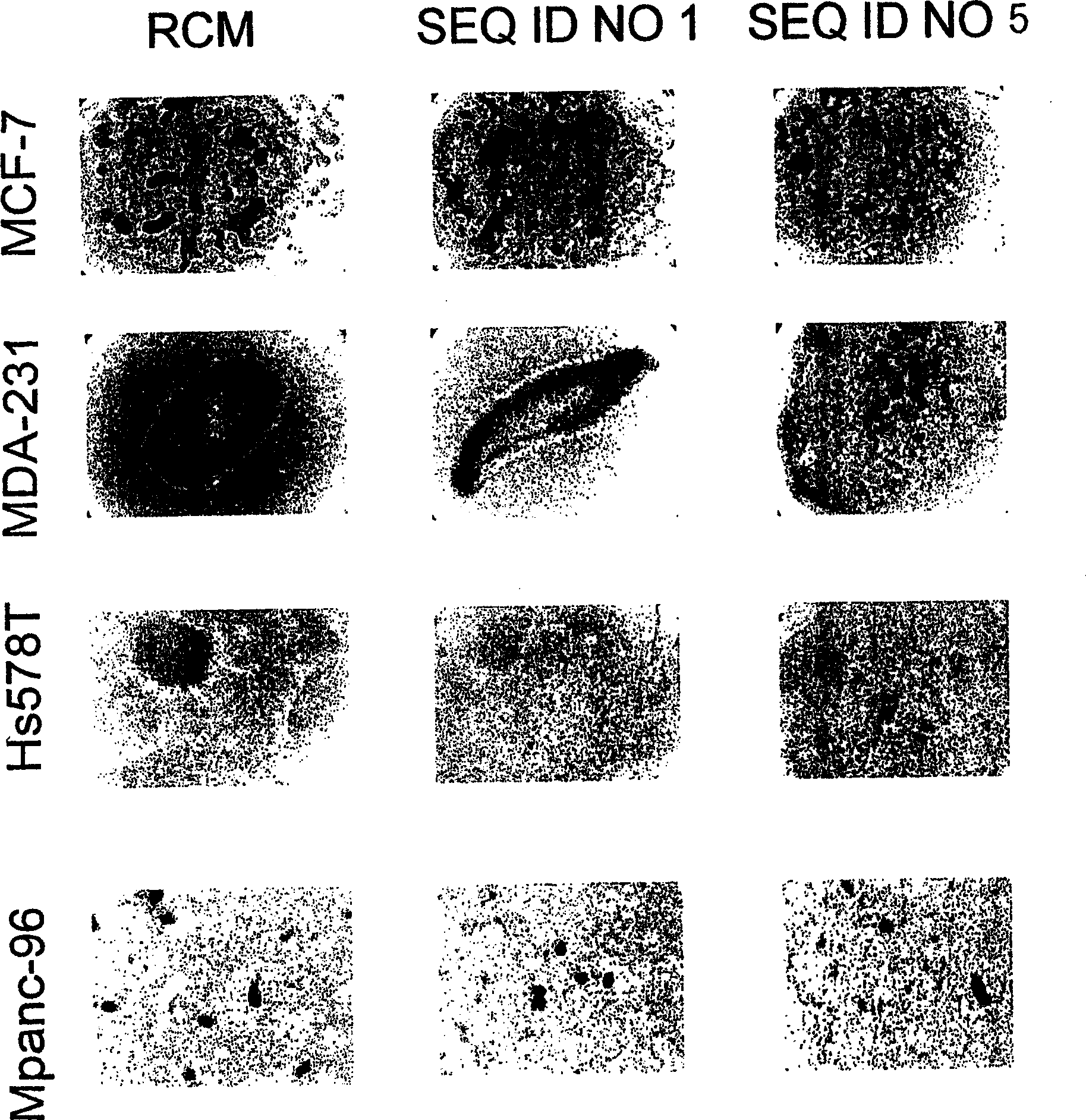
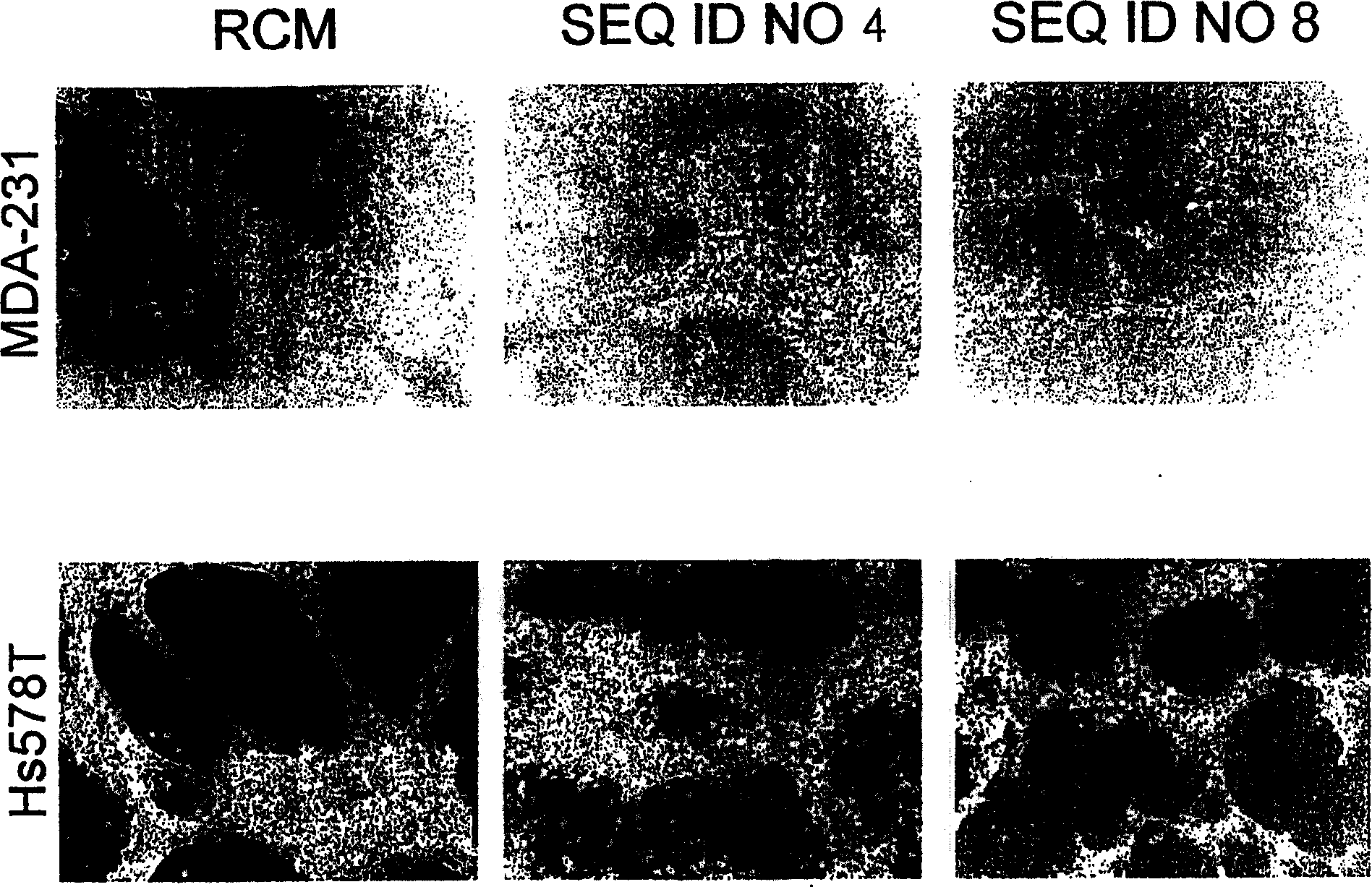
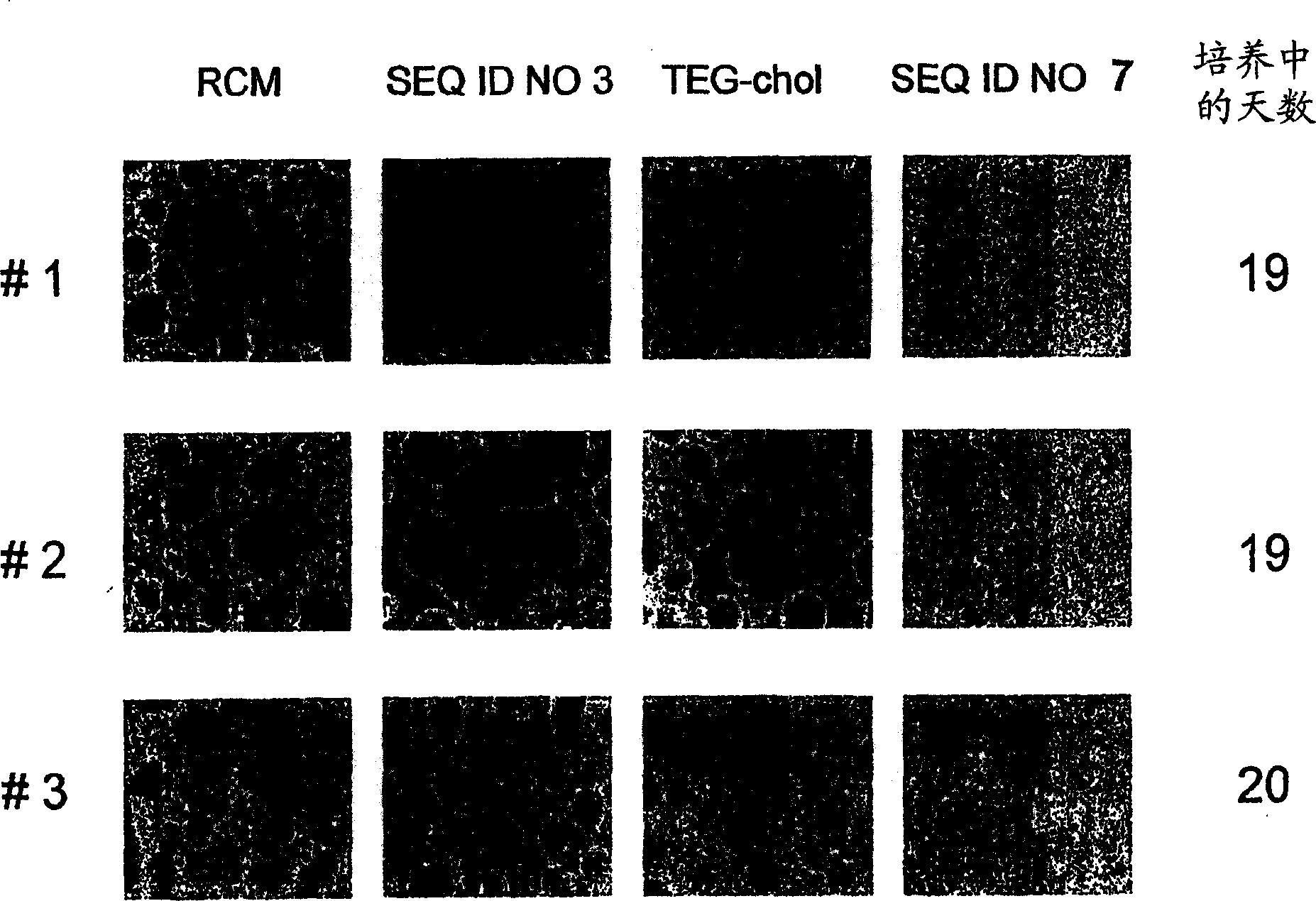
Embodiment 3
[0080] Apoptosis in MEG-01 cells was induced by SEQ ID NO:5 and SEQ ID NO:6.
[0081] Redistribution of plasma membrane phosphatidylserine is a characteristic of cells undergoing apoptosis (Martin et al., J. Exp. Med., 182:1545, 1995). The redistribution of phosphatidylserine in the plasma membrane during apoptosis was determined by flow cytometry using FITC (fluorescein isothiocyanate)-conjugated annexin V (BD Pharmingen, San Diego, CA). technically determined. The human chronic myeloid leukemia cell line MEG-01 cells that are positive for Philadelphia chromosome and have BCR-ABL gene fusion were used as 2.5×10 5 Concentration of cells / ml vs. final concentrations of 5.3, 26.5 and 53.0 μM of SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 5, SEQ ID NO: 6 and cholestenyl-TEG phosphoramidite molecules Incubate together for 48 hours. The percentage of cells undergoing apoptosis following treatment with SEQ ID NOs: 1, 2, 5, 6, or cholestenyl-TEG phosphoramidites is reported in Table 2. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com