Gene engineering bacteria of high efficiency expression of human alpha 1-thymulin and its construction method and use
A technology of genetically engineered bacteria and high-efficiency expression, applied in genetic engineering, medical preparations containing active ingredients, applications, etc., to achieve low production costs, simple purification steps, and increased expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
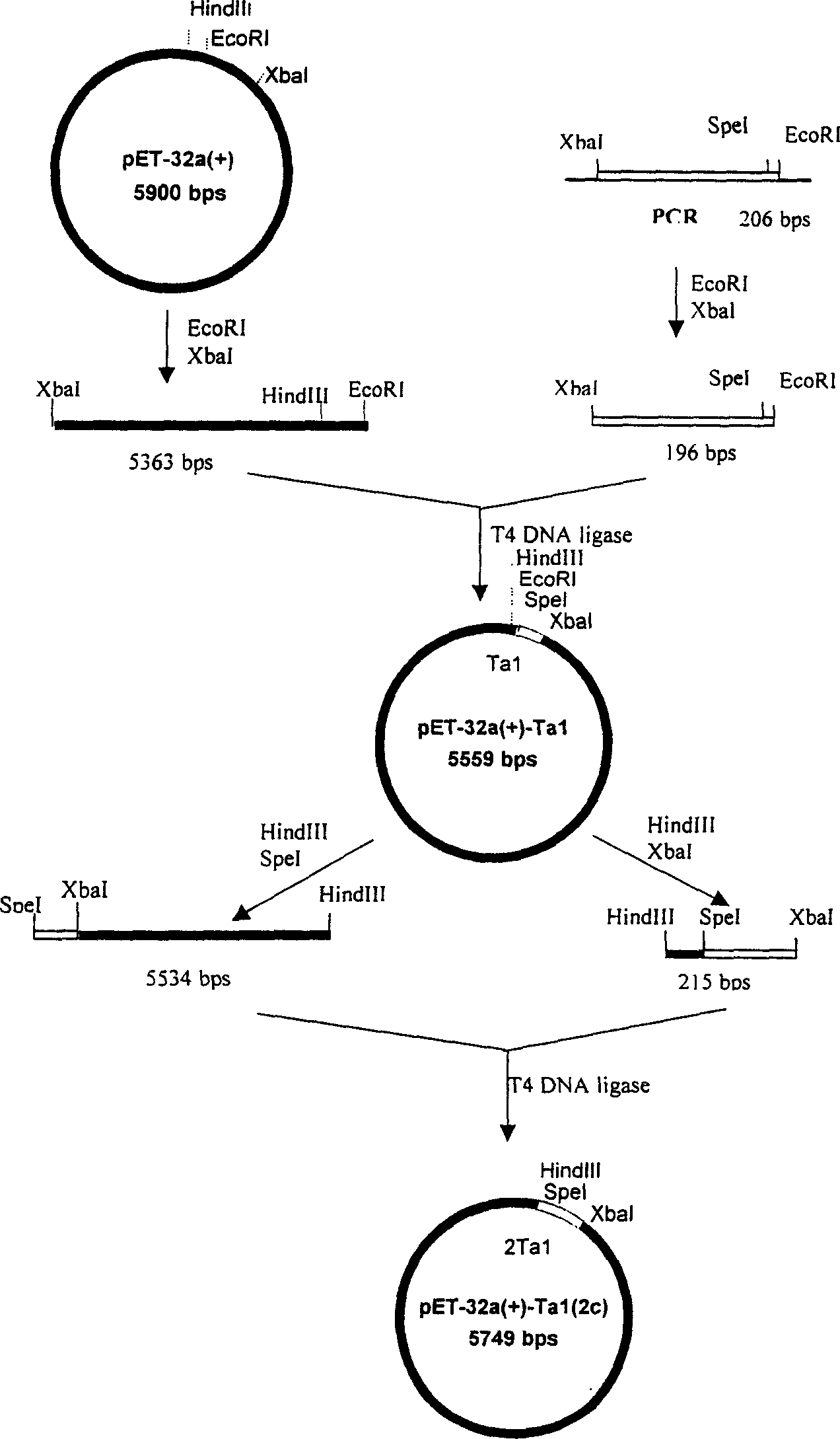
[0038] Example 1 Construction of a genetically engineered bacterium that highly expresses human α1-thymosin: it contains 1-16 SD-His·Tag-EK-Tα 1 -Genetically engineered bacteria of TAA tandem units. The following pET32a(+) plasmid was purchased from Novagen.
[0039] The first step base sequence synthesis
[0040] According to the amino acid sequence of human α1-thymosin, the Escherichia coli preferred codon was selected to synthesize the following 206bp fragment:
[0041] XbaI SD-Sequence
[0042] 5'-CC tctaga AATAATTTTGTTTAACTTTAAG AAGGAGA TATACATATGTCTGGA
[0043] Met Ser Gly
[0044] His Tag KpnI
[0045] TCAGGT CATCATCATCATCATCAT TCTTCT ggtacc GATGACGACGACAAG
[0046] Ser Gly His His His His His His His Ser Ser Gly Thr Asp Asp Asp Asp Lys
[0047] EK
[0048] AGCGATGCCGCCGTGGATACCAGCAGCGAAATTACCACCAAAGATCTGAAA
[0049] Ser AspAla Ala V...
Embodiment 2
[0058] Example 2 Construction of a genetically engineered bacterium that highly expresses human α1-thymosin: Contains 1-16 P-SD-His·Tag-EK-Tα 1 -Genetically engineered bacteria of TAA tandem units. The P is a promoter (Promotor, P).
[0059] The first step base sequence synthesis
[0060] With embodiment 1.
[0061] The second step is to construct a single P-SD-His·Tag-EK-Tα 1 -Genetically engineered bacteria with TAA DNA sequence
[0062] Extract the pMD18-T plasmid, purify and recover small fragments after double digestion with XbaI / EcoRI; meanwhile, digest plasmid pET-32a(+) with XbaI / EcoRI double digestion, and recover large fragments. The recovered product was ligated and transformed into Escherichia coli DH5α for amplification. A single string of P-SD-His·Tag-EK-Tα was obtained 1 - Plasmid pET-32a-Tα for the TAA gene 1 -1c (1c represents the fusion gene monomer, 2c represents the fusion gene double body, and so on).
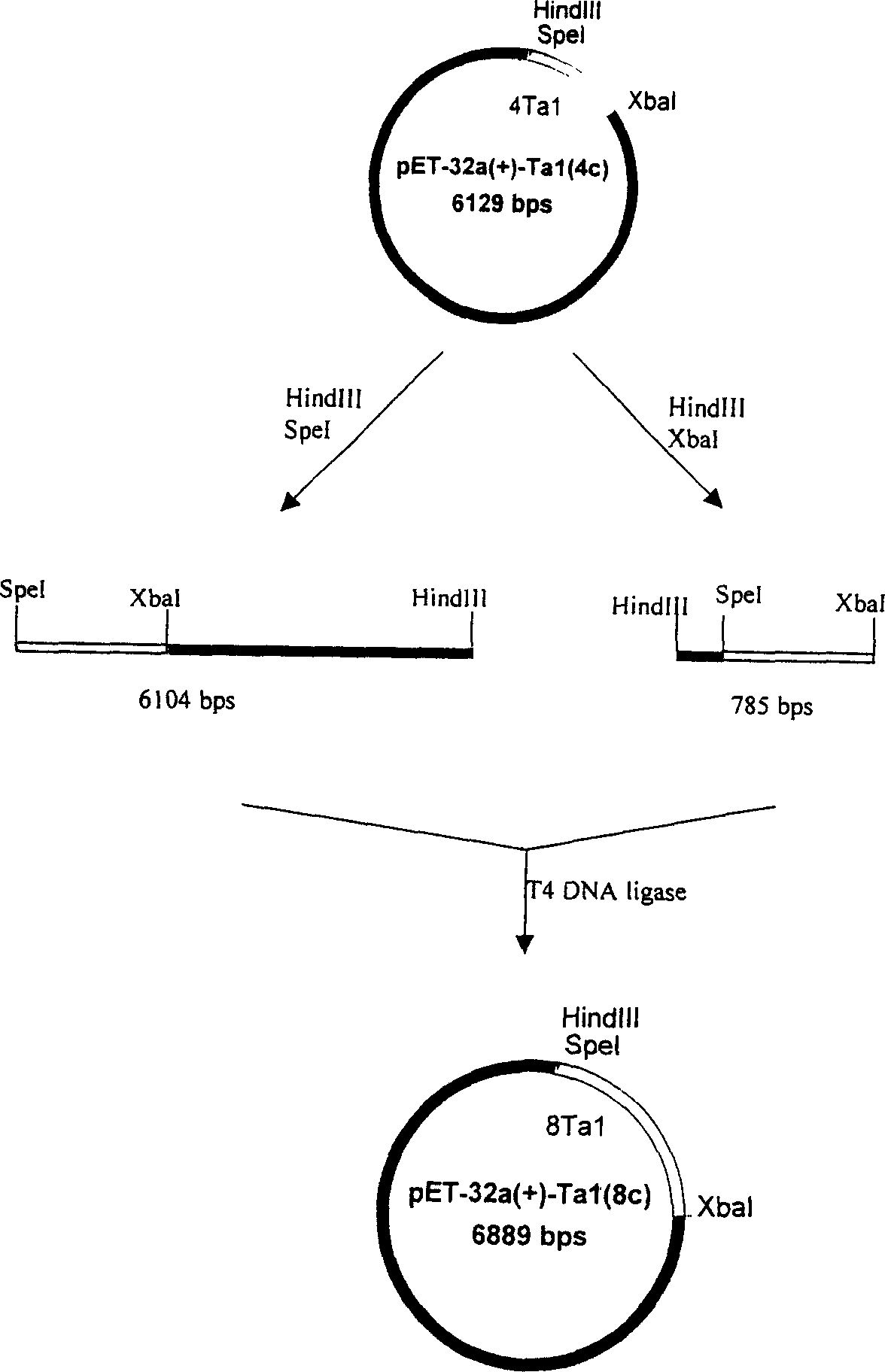
[0063] The third step is to construct multipl...
Embodiment 3
[0066] Example 3 Construction of a genetically engineered bacterium that highly expresses human α1-thymosin: containing 1-16 SD-TrxA-His·Tag-EK-Tα 1 -Genetically engineered bacteria of TAA tandem units. The TrxA is thioredoxin.
[0067] The first step base sequence synthesis
[0068] With embodiment 1.
[0069] The second step is to construct a single SD-TrxA-His·Tag-EK-Tα 1 -Genetically engineered bacteria with TAA DNA sequence
[0070] The pMD18-T plasmid was extracted, and the small fragment was purified and recovered after double digestion with KpnI / EcoRI; at the same time, the large fragment was recovered by double digestion of the plasmid pET-32a(+) with KpnI / EcoRI. The recovered product was ligated and transformed into Escherichia coli DH5α for amplification. Since the plasmid pET-32a(+) is a thioredoxin fusion protein expression system, a single string of SD-TrxA-His·Tag-EK-Tα can be obtained 1 - Plasmid pET-32a-TrxA-Tα for the TAA gene 1 -1c (1c represents the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com