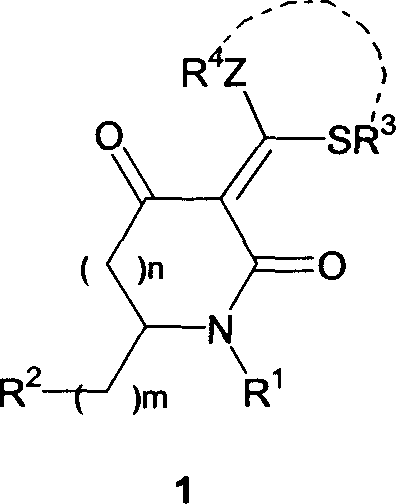
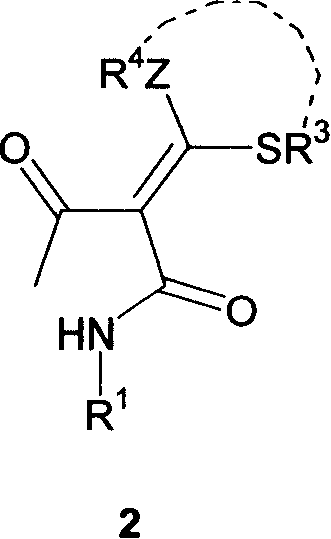
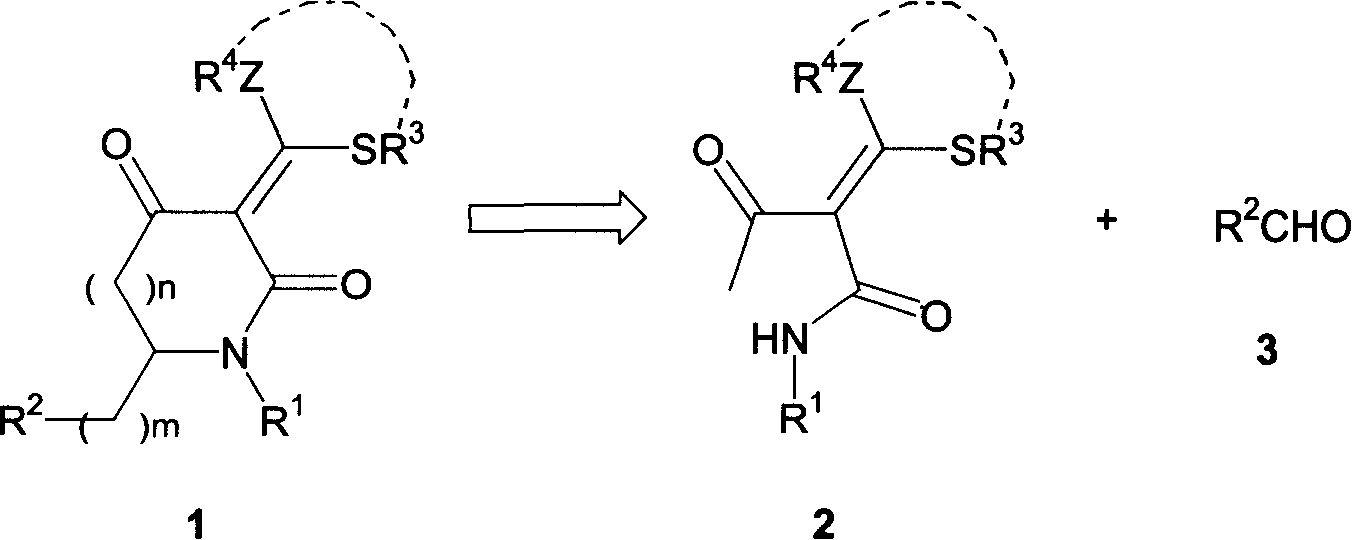
Substituded acza nember 5/nember 6 ring compound and its preparation process
A technology of compounds and benzene rings, applied in the direction of organic chemistry, etc., can solve the problems of many steps, lack of product diversity, and complicated operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 100 ml Erlenmeyer flask, add 50 ml of DMF, 15.2 g (110 mmol) of anhydrous K 2 CO 3 , stirred for 10 minutes, then added amine / carbamoylacetone (500mmol), stirred for 0.5 hours, added 4 milliliters (55mmol) of carbon disulfide under ice cooling and continued to stir for 1 hour, added dihalogenated hydrocarbon (55mmol), stirred for 10 hours, poured into 150 ml of water, a large amount of white solid was precipitated, suction filtered, and dried to obtain a light yellow solid product α-ammonia / carbamoyl dithioketal 2. The reaction is shown in the following formula, and the data is shown in the table below:
[0021]
[0022] R 1 =H; R, R=(CH 2 ) 2 77.2%
[0023] R 1 =H; R, R=(CH 2 ) 3 78.5%
[0024] R 1 =H; R, R=(CH 2 ) 4 76.0%
[0025] R 1 =H; R=CH 3 73.2%
[0026] R 1 =H; R=Bn 72.0%
[0027] R 1 =ClC 6 h 4 ; R, R=(CH 2 ) 2 93.0%
[0028...
Embodiment 2
[0038] In a 100 ml Erlenmeyer flask, add 50 ml of DMF, 15.2 g (110 mmol) of anhydrous K 2 CO 3 , stirred for 10 minutes, then added 6.4 milliliters (500 mmol) of ethyl acetoacetate, stirred for 0.5 hours, added 4 milliliters (55 mmol) of carbon disulfide under ice cooling and continued to stir for 1 hour, added 1,3-dibromopropane (or 1,2- Dibromoethane and 1,4-dibromobutane) (55mmol), stirred for 10 hours, poured into 150 milliliters of water, a large amount of white solid was precipitated, filtered by suction, and dried to obtain the white solid product 2-[1,3] Ethyl dithio-2-methylene-3-oxobutyrate, yield 85.0%. The reaction is shown in the following formula, and the data are shown in the table below.
[0039]
[0040] R, R=(CH 2 ) 2 93.8%
[0041] R, R=(CH 2 ) 3 95.0%
[0042] R, R=(CH 2 ) 4 91.3%
[0043] R=CH 3 87.5%
[0044] R=Bn 86.2%
[0045] According to the hydroly...
Embodiment 3
[0056] Ethyl 2-dialkylthiomethylene-3-carbonylbutanoate (5 mmol) synthesized in Example 2 and aromatic aldehyde ArCHO (6 mmol) were added to ethanol (15 mL) and stirred in an ice-water bath for 5 min. 1N NaOEt / EtOH solution (6mL, equivalent to 6mmol EtONa, prepared now) was added dropwise, and stirred at room temperature. TLC detects that the reaction causes the disappearance of the substrate (it takes about 4 h). The solvent ethanol was evaporated under reduced pressure, and the residual solid after evaporation of ethanol was quickly washed with hydrochloric acid (10 mL, 1N), filtered with suction, washed with water, and dried to obtain a yellow solid -α-cinnamoyl-α-carboxy dithioketene.
[0057] Pour dry CH into a 50-ML dry three-necked bottle 2 Cl 2 (or THF, 10mL) and compound α-cinnamoyl-α-carboxydithioketene (2.0mmol), after stirring for 15min, add SOCl 2 (285.6mg, 2.4mmol), heated to reflux in a hot water bath for 3min under electromagnetic stirring, and stopped heati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com