Macrocyclic peptides active against the hepatitis C virus
A hepatitis virus, cycloalkyl technology, applied in the field of compounds, can solve problems such as reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
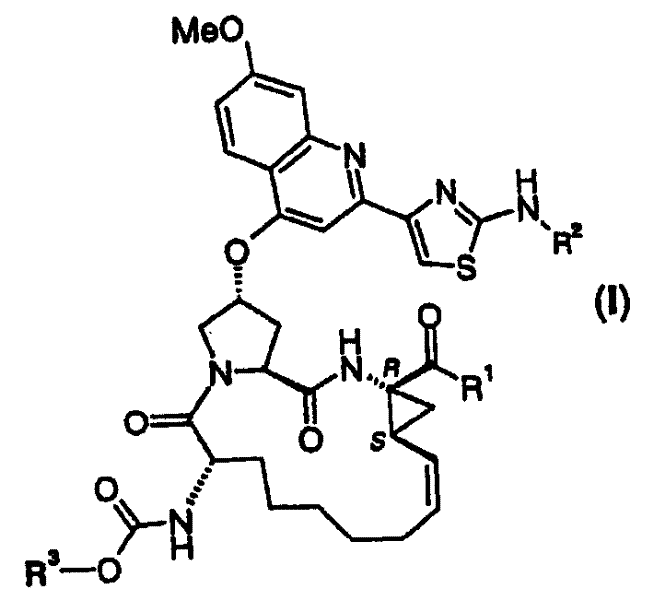
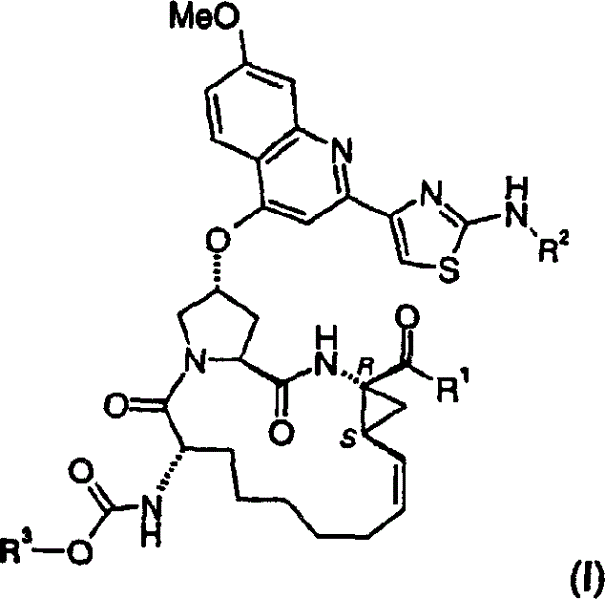
[0071] Preferably, the above-mentioned compound of formula I, wherein R 1 is hydroxyl or NHSO 2 R 1A , where R 1A Yes (C 1-6 ) alkyl, (C 3-7 ) cycloalkyl or {(C 1-6 ) Alkyl-(C 3-7 )cycloalkyl}, which are optionally halogen, nitro or O-(C 1-6 ) Alkyl is substituted 1-3 times, or phenyl, which is optionally replaced by halogen, nitro, (C 1-6 ) Alkyl or O-(C 1-6 ) alkyl groups are substituted 1 to 3 times.
[0072] More preferred are compounds of I above, wherein R 1 is hydroxyl or NHSO 2 R 1A , where R 1A is methyl, ethyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclopropylmethyl, cyclohexylethyl, CCl 3 , CF 3 , phenyl, 2-fluorophenyl or 4-methylphenyl.
[0073] Most preferred are compounds of formula I as defined above, wherein R 1 is hydroxyl or NHSO 2 R 1A ; where R 1A is methyl, cyclopropyl, CF 3 or phenyl. Yujia R 1A For cyclopropyl.
[0074] R 1 Most preferred is hydroxyl.
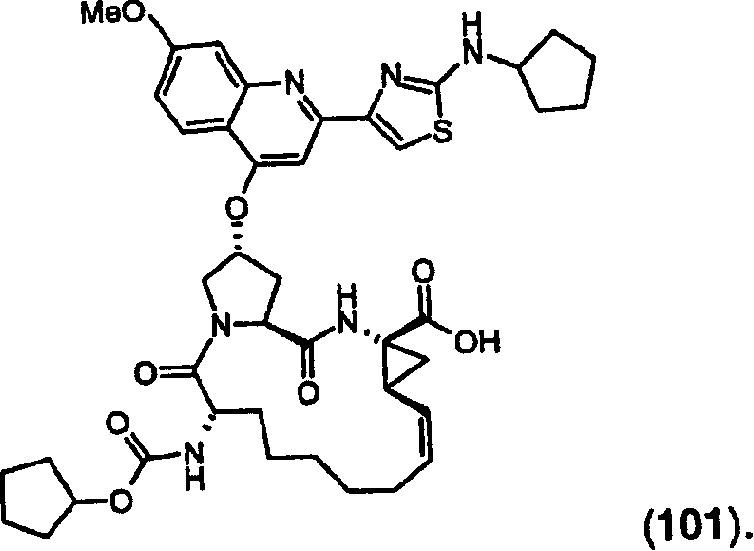
[0075] R 2 Most preferred is cyclopentyl.
[0076] All compounds of formul...
Embodiment 1
[0110] Synthesis of dipeptide 1c
[0111]
[0112] Boc-hydroxyproline 1a (50.0 g, 216 mmol), (1R, 2S)-vinyl-ACCA hydrochloride 1b (42.25 g, 238 mmol), TBTU (76.36 g, 238 mmol) and A mixture of DIPEA (113 mL, 649 mmol) in DMF (800 mL) was stirred at room temperature under nitrogen. After 3.5 hours, the solvent was evaporated and the residue was extracted with EtOAc. The extract was washed with hydrochloric acid (10%), saturated sodium bicarbonate and brine. The organic phase was then dried over magnesium sulfate, filtered and evaporated to an oil. After drying under high vacuum overnight (18 hours), dipeptide 1c was obtained as a yellow foam (72.0 g, 94%, purity >95% by HPLC).
Embodiment 2
[0114] Synthesis of dipeptide 2a
[0115]
[0116] Dipeptide 1c (72.0 g, 203 mmol), triphenylphosphine (63.94 g, 243.8 mmol, 1.2 eq) and 4-nitrobenzoic acid (41.08 g, 245.8 mmol, 1.2 eq) were dissolved in anhydrous THF (1.4 L). The stirred solution was cooled to 0 °C under nitrogen. DEAD (38.4 mL, 244 mmol, 1.2 equiv) was then added dropwise over 45 minutes and the reaction was allowed to warm to room temperature. After 4 hours, the solvent was evaporated and the residue was divided into four portions. Each aliquot was prepared on fine silica gel (10-40 micron mesh, column diameter 12 cm, column length 16 cm) with a gradient of 2:1 hexane / EtOAc to 1:1 hexane / EtOAc to pure EtOAc. Chromatographic eluent purification. Ester 2a was obtained as an amorphous white solid (108.1 g, quantitative yield) after evaporation of the solvent and drying under high vacuum at 70°C for 1 hour.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com