Liquid preparation containing penta peptide of thymus and preparing method
A liquid preparation, thymus technology, applied in anti-inflammatory agents, pharmaceutical formulations, antiviral agents, etc., can solve problems such as high cost, instability of polypeptide drugs affecting patient treatment, and drugs failing to reach therapeutic concentrations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
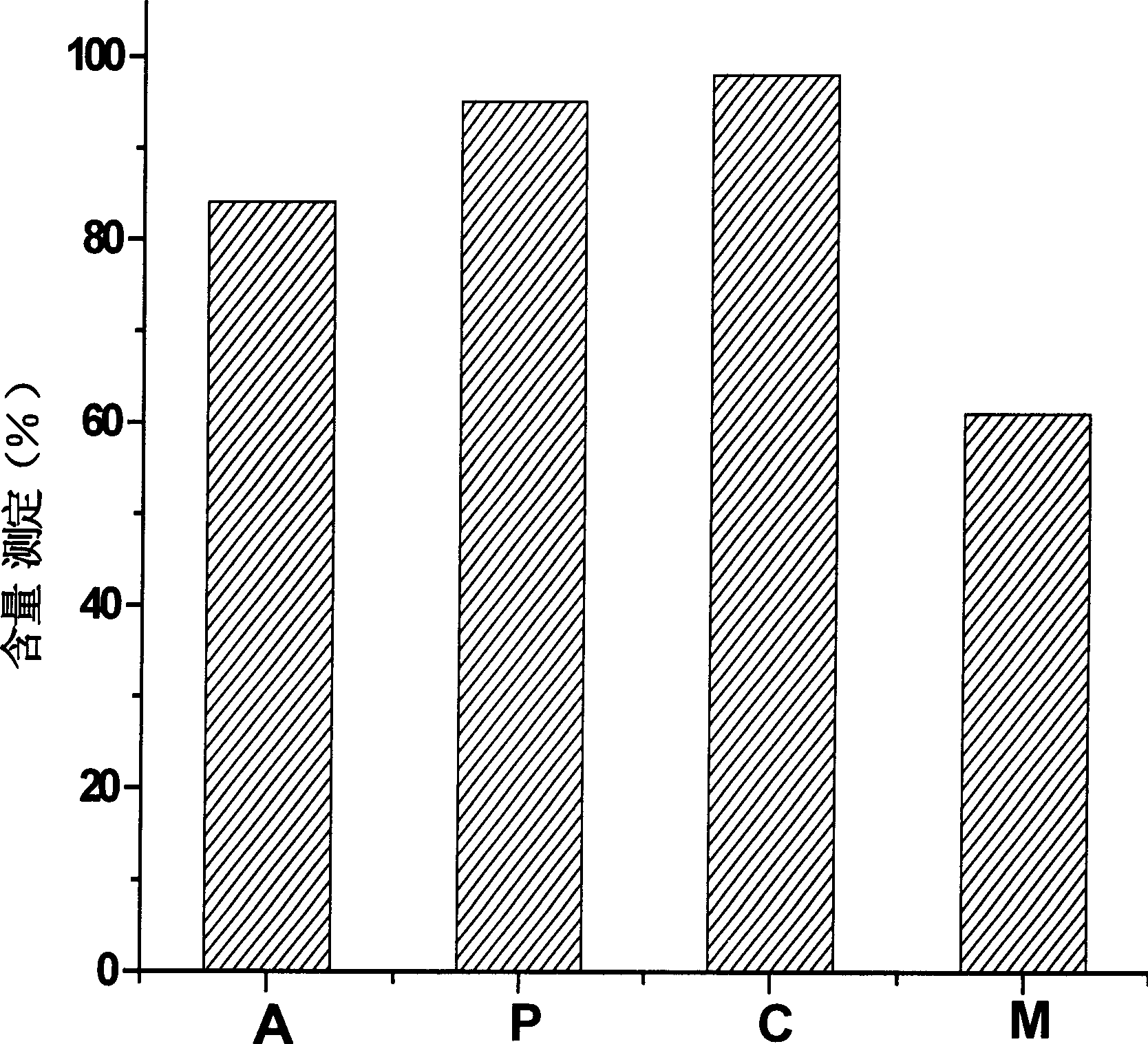
[0017] Example 1, buffer solution on the effect of Thymopentin stability
[0018] Thymopentin solutions were prepared according to the buffer solutions listed in Table 1, with a concentration of 1 mg / ml. The resulting solution was sterilized by filtration with a microporous membrane to prepare 1 ml / support of injections. Put the sample into a thermostat at 45°C, and measure the content of the sample on the 0th day and the sample on the 10th day according to the following method.
[0019] sample
buffer
A
30mM acetate buffer, 5% mannitol, pH7.2
P
30mM Phosphate Buffer, 5% Mannitol, pH7.2
C
30mM citrate buffer, 5% mannitol, pH7.2
M
5% Mannitol, pH7.2
[0020] The content determination method adopts high-performance liquid chromatography, using octadecyl bonded silica gel as a filler, and phosphate buffer-methanol (60:40) at pH 7.0 as a mobile phase; the detection wavelength is 275nm. The num...
example 2
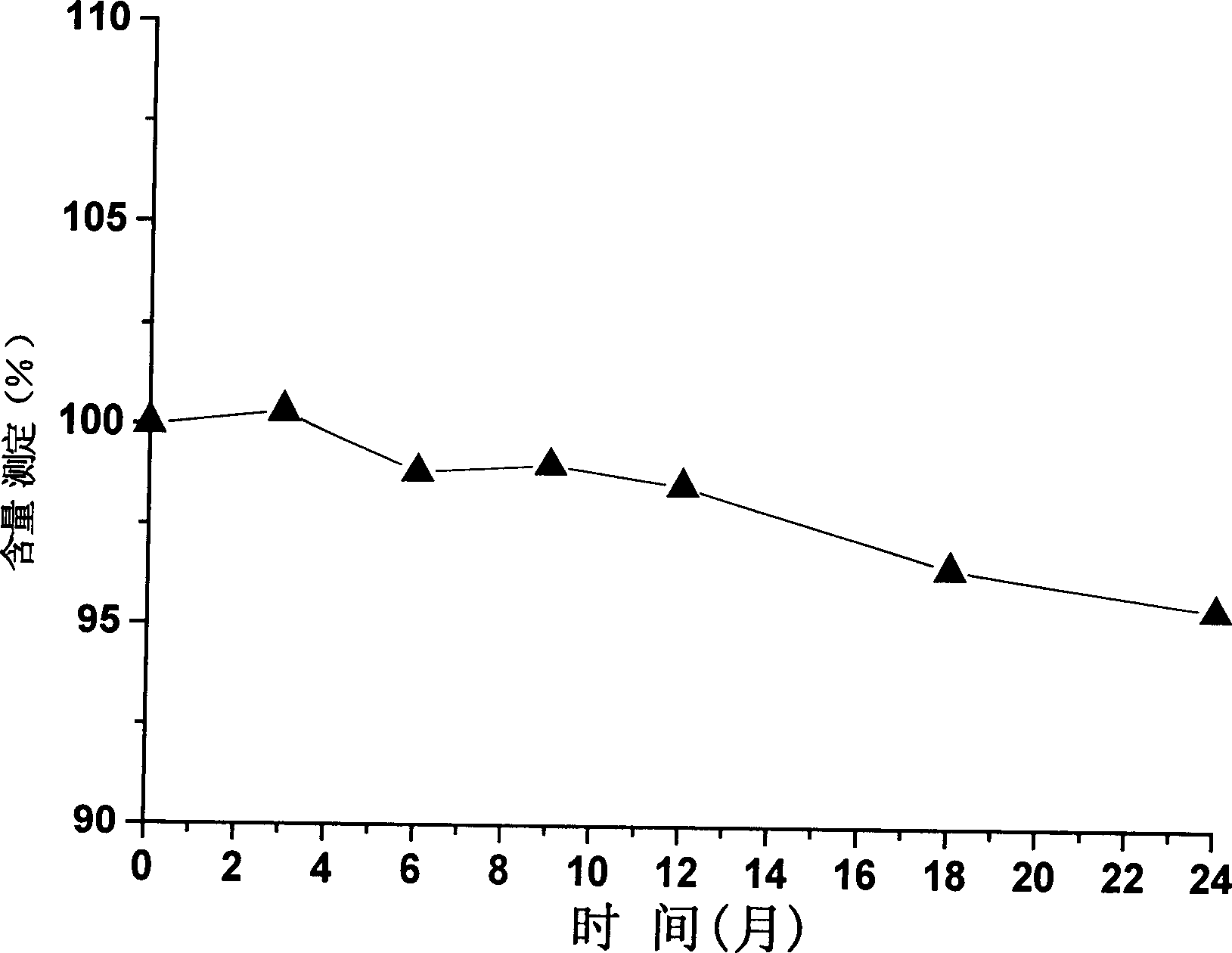
[0022] Example 2, the effect of the citrate buffer containing sodium chloride on the stability of thymopentin
[0023] In order to further investigate the stability of thymopentin in refrigerated conditions (4°C) in citrate buffer containing sodium chloride, a 1mg / ml thymopentin liquid preparation (pH7.2) was prepared, which contained 30mM lemon Salt buffer and 118mM NaCl, NaCl is the osmotic regulator. Store at 4°C and observe for 24 months, and measure the content of thymopentin at the 0th month, 3rd month, 6th month, 9th month, 12th month, 18th month, and 24th month according to the content determination method.
[0024] Based on the content of the sample at month 0 as 100%, calculate the content determination percentage of the sample at each time point. figure 2 Shows the results of the 24-month stability test of the sample at 4°C. The results showed that: under the condition of 4℃, the content of thymopentin in the citrate buffer containing sodium chloride had no signi...
example 3
[0025] Example 3, the preparation of the liquid formulation containing citrate buffer
[0026] Prescription: Thymopentin 1.0g
[0027] Sodium citrate 6.9g
[0028] Sodium chloride 8.8g
[0029] Add water for injection to 1000ml
[0030] Operation steps: Take about 600ml of water for injection and put it into an appropriate container, add 6.9g of sodium chloride and 8.8g of sodium citrate and stir until the solution is clear. Then 1.0 g of thymopentin was added under stirring (to prevent foaming) until all dissolved and clarified. The resulting solution was adjusted to pH 7.0-7.4 with 0.1M NaOH or 0.1M HCl. Add water for injection to 1000ml, stir until completely clear. The obtained solution is sterilized by filtration with a microporous filter membrane, and then packaged to make 1ml / support injections.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com