Application of camptothecin derivative in drug preparing process for treating marrow originate leukemia
A technology for myeloid leukemia and derivatives is applied in the application field of camptothecin derivatives in the preparation of drugs for the treatment of myeloid leukemia, and achieves the effects of good medicinal prospects and strong pharmacological effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Application of camptothecin derivative NSC606985 in preparation of medicine for treating myeloid leukemia
[0050] Step 1. Cell culture and processing
[0051] Acute promyelocytic leukemia NB4 cells (provided by Dr. Michael Lannotte), acute monocytic leukemia U937 cells and chronic myelogenous leukemia K562 cells (from the cell bank of Shanghai Institute of Biological Sciences, China) were all incubated at 37°C, 5% Under the condition of CO2-95% air humidity, culture was carried out in RPMI-1640 medium containing 10% heat-inactivated fetal calf serum (FCS, Hyclone, Logan, UT). 2-5×10 experimental cells 5 cells / ml and incubated with desired concentrations of NSC606985 in the presence / absence of the PKCδ-specific inhibitor rottelin and the caspase-3 inhibitor z-DEVD-fmk. NSC606985 (provided by the National Cancer Institute Anticancer Drug Screening Standard Drug Database) was dissolved in double distilled water and prepared as a 25 μM stock solution. Rottlerin (BIOMOL,...
Embodiment 2
[0061] Evaluation of the effect of camptothecin derivative NSC606985 on myeloid leukemia
Embodiment 2-1
[0063] Nanomolar concentration of NSC606985 induces apoptosis in promyelocytic leukemia cell lines
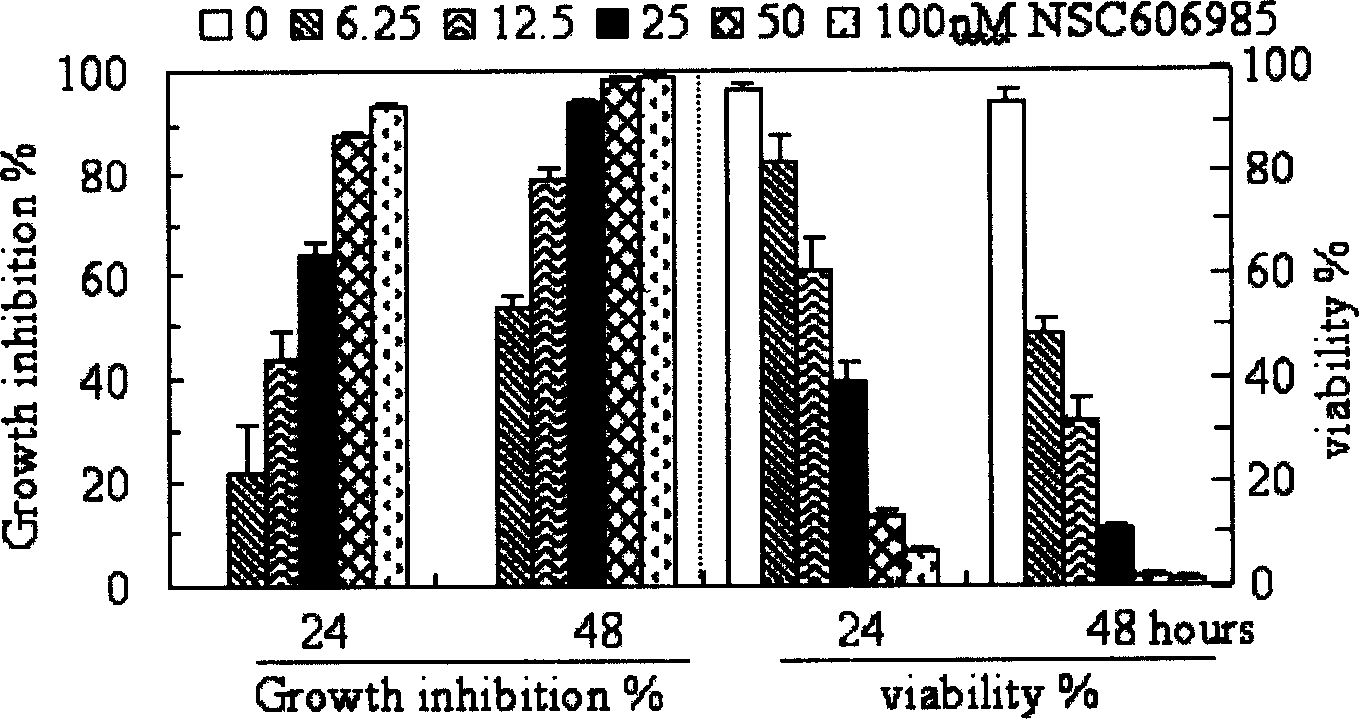
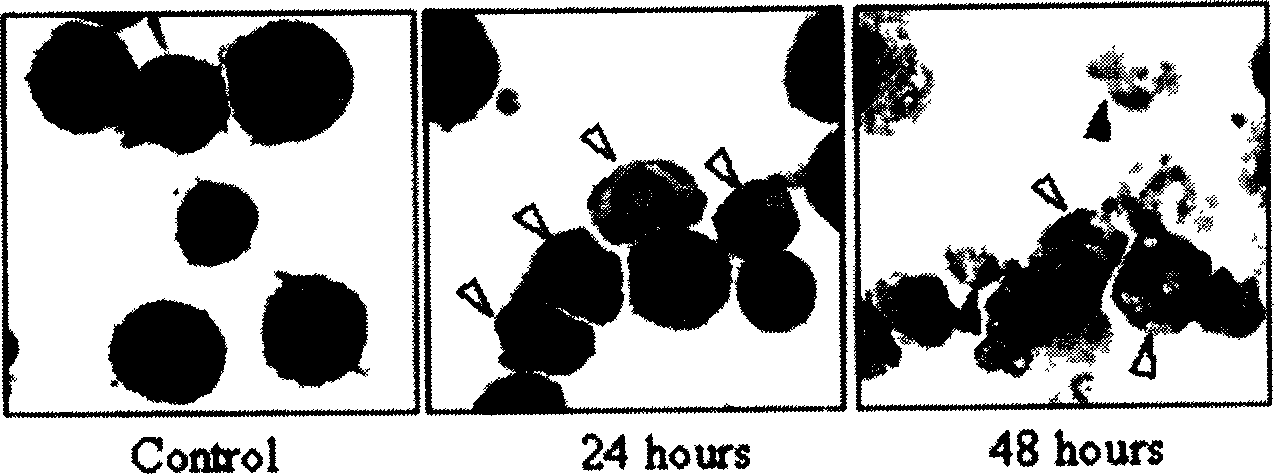
[0064] In the APL cell line NB4 cells, NSC606985 inhibited cell growth in a time-dose dependent manner ( Figure 1B , left), which is consistent with decreased cell viability ( Figure 1B ,right). This inhibitory effect was observed at nanomolar concentrations. After 48 hours of treatment, 6.25nM NSC606985 caused 48.2 ± 2.8% cell death, and 50nM and 100nM NSC606985 caused almost all cell death ( Figure 1B ,right). Further evidence supports that NSC606985 induces cell death through apoptosis. NB4 cells treated with NSC606985 showed profound cell morphological changes, such as chromosome condensation, nuclear rupture without cell membrane damage and other apoptosis characteristics ( Figure 1C ). Analysis of nuclear DNA content by flow cytometry showed that after NSC606985 induction, due to the degradation and leakage of nuclear DNA, hypoploid cells (also known as sub-G1 c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com