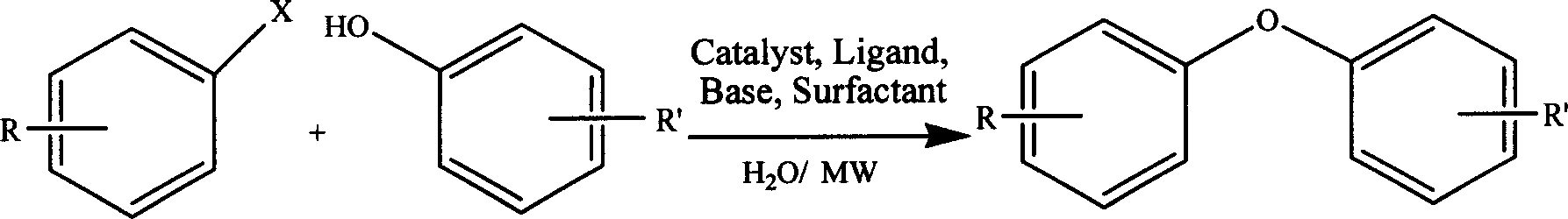
Water heating synthesis of diaryl ether
A technology of diaryl ether and synthetic method, which is applied in ether preparation, organic chemistry, etc., can solve the problems that hinder the application of high-throughput biological activity screening, long reaction time, and difficult handling, so as to improve environmental friendliness and reaction time The effect of shortening and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of 4-nitrophenylphenyl ether
[0023] 101mg (0.5mmol) 4-nitrobromobenzene, 56.4mg (0.6mmol) phenol, 104mg (0.75mmol) K 2 CO 3 , 32mg (0.1mmol) TBAB, 1.5ml H 2 O was added to an 8ml microwave reaction tube, and reacted at 100W and 110°C for 10min. After the reaction stopped, it was extracted with ethyl acetate, washed with water, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was distilled under reduced pressure, separated and purified by silica gel column chromatography [eluent: petroleum ether / ethyl acetate ( 10:1)], 82 mg of 4-nitrophenylphenyl ether was obtained, and the yield was 70%.
[0024] product 1 H NMR (CDCl 3 , 300MHz) δ7.00(m, 2H), 7.08(2, 2H), 7.24(t, J=7.8Hz, 1H), 7.42(t, J=7.8Hz, 2H), 8.18(m, 2H).
Embodiment 2
[0025] Embodiment 2: the synthesis of 4-nitrophenyl-4'-chlorophenyl ether
[0026] 101mg (0.5mmol) 4-nitrobromobenzene, 128mg (1mmol) p-chlorophenol, 11mg (0.05mmol) CoAc 2 4H 2 O, 26.2 mg (0.1 mmol) PPh 3 , 104 mg (0.75 mmol) K 2 CO 3 , 32mg (0.1mmol) TBAB, 1.5ml H 2 O was added to an 8ml microwave reaction tube, and reacted at 100W and 170°C for 20min. After the reaction stopped, it was extracted with ethyl acetate, washed with water, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was distilled under reduced pressure, separated and purified by silica gel column chromatography [eluent: petroleum ether / ethyl acetate ( 10:1)] to obtain 115 mg of 4-nitrophenyl-4'-chlorophenyl ether with a yield of 92%.
[0027] product 1 H NMR (CDCl 3 , 300MHz) δ7.01 (m, 4H), 7.38 (d, J=8.7, 2H), 8.20 (d, J=9.3, 2H).
Embodiment 3
[0028] Embodiment 3: the synthesis of phenylphenyl ether
[0029] With 79mg (0.5mmol) bromobenzene, 94mg (1mmol) phenol, 20mg (0.1mmol) PdAc 2 , 12mg (0.1mmol) TMEDA, 104mg (0.75mmol) K 2 CO 3 , 32mg (0.1mmol) TBAB, 1.5ml H 2O was added to an 8ml microwave reaction tube, and reacted at 100W and 190°C for 90min. After the reaction stopped, it was extracted with ethyl acetate, washed with water, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was distilled under reduced pressure, separated and purified by silica gel column chromatography [eluent: petroleum ether] to obtain phenyl Phenyl ether 31 mg, yield 37%.
[0030] product 1 H NMR (CDCl 3 , 300MHz) δ6.98 (m, 4H), 7.07 (m, 2H), 7.30 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com