Dendron shaped cell tumour vaccine for loading withered heat shock tumour cell, its preparation method and application
A technology of dendritic cells and tumor cells, applied in the field of biopharmaceuticals, can solve the problems of increasing pollution control difficulty, tediousness, complicated process, etc., and achieve the effect of maintaining antigenicity, simple operation, and efficient induction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: MDDC tumor vaccine loaded with apoptotic heat shock melanoma cells Me290
[0072] 1. Heat shock treatment of melanoma cells Me290
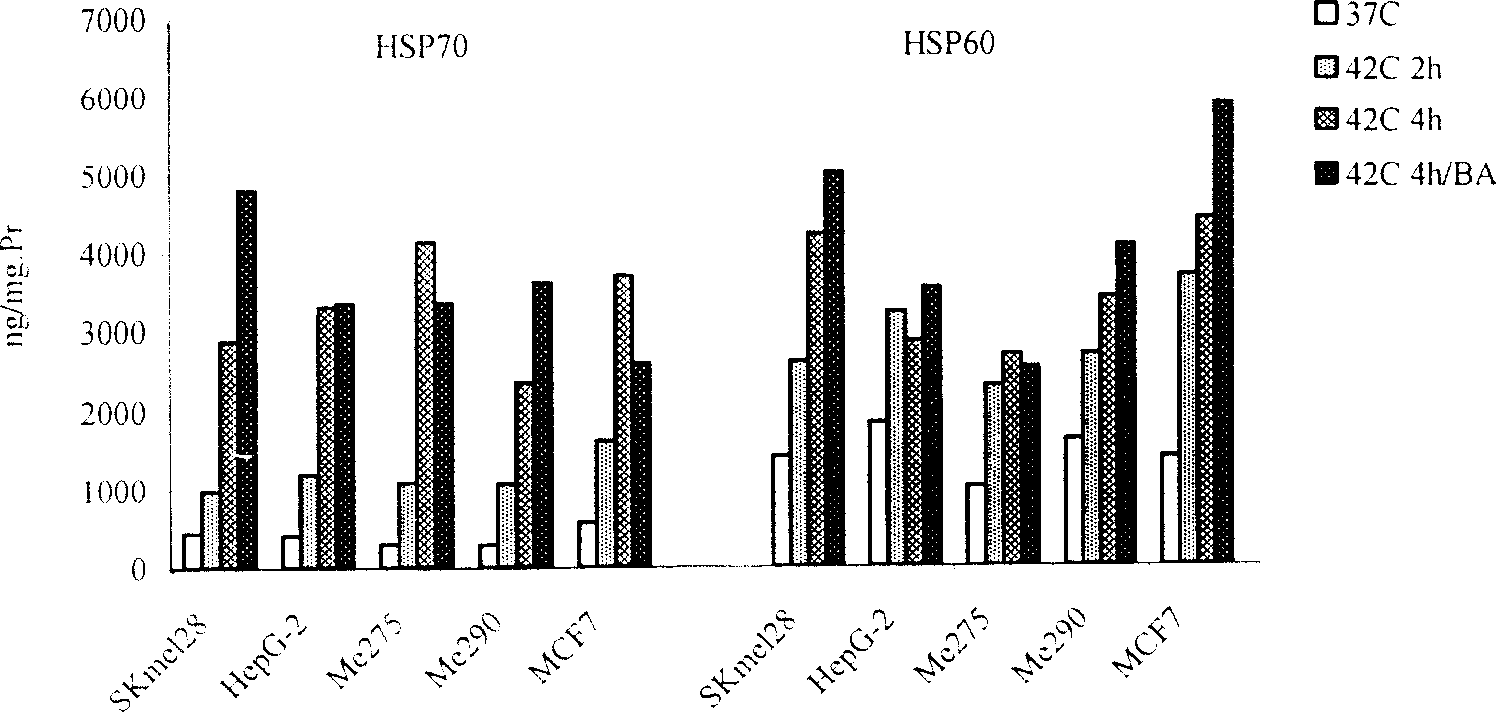
[0073] Inoculate melanoma cells Me290 in RPMI1640 cell culture medium with 10% FCS, and move the cells to 5% CO 2 , 42°C cell culture incubator or 42°C water bath, heated for 2h.
[0074] 2. Induction of apoptosis in Me290 cells after heat shock
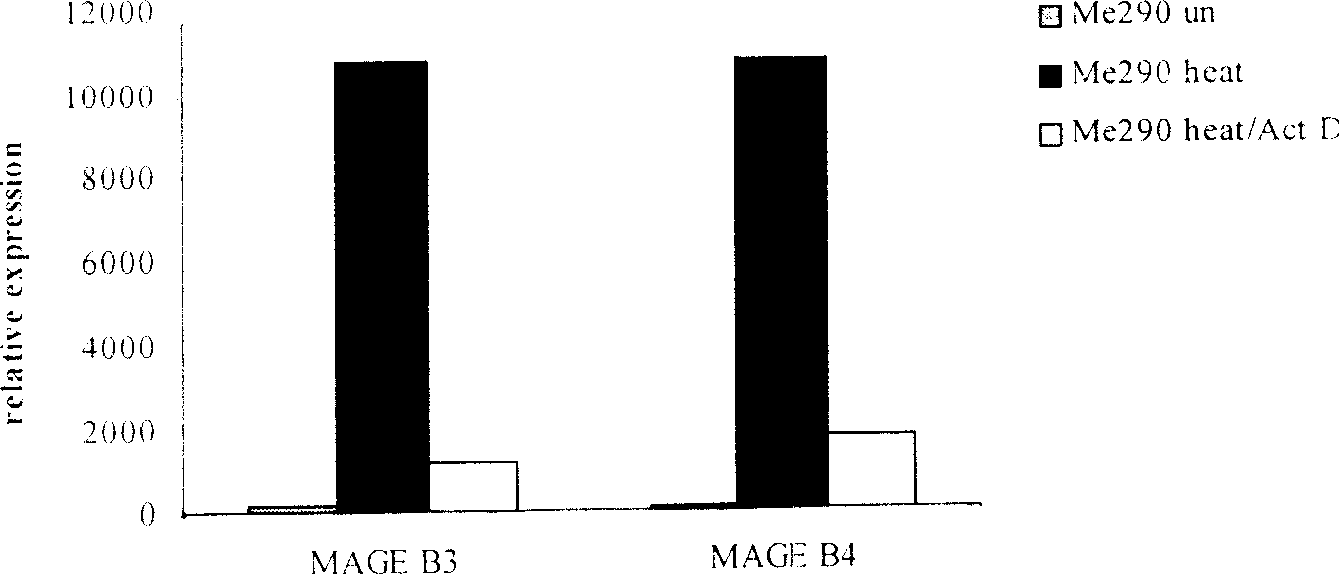
[0075] Move the Me290 cells after the above heat shock back to 5% CO 2 , continue to cultivate in the cell incubator at 37°C, add BA (an anticancer drug extracted from plants, purchased from Sigma Company) at the same time, and the effect concentration is 10ug / ml, after continuing to cultivate for 48h, collect the apoptotic tumor cells by centrifugation, and use Washed with PBS for 3 times, and finally the collected tumor cells were suspended in RPMI1640 medium and irradiated with 100GY of γ-rays before use.
[0076] 3. Induction of MDDCs in vitro (dendritic cells derived from monoc...
Embodiment 2
[0080] Example 2: MDDC Tumor Vaccine Loaded with Apoptotic Heat Shock Liver Cancer Cells
[0081] 1. Processing of Liver Cancer Samples
[0082] Take fresh liver cancer tissue (operation or puncture) and place it in a sterile container immediately. The preservation solution is freshly prepared RPMI1640 medium containing double antibody (penicillin / streptomycin). The shredded liver cancer tissue pieces were repeatedly ground in a sterile syringe, and finally the cells were suspended in the preservation solution and filtered through a 200-mesh filter to prepare a single-cell suspension.
[0083] 2. Heat shock liver cancer cells
[0084] The liver cancer cell suspension prepared above was inoculated in RPMI1640 cell culture medium containing 2% mixed human AB serum in 5% CO 2 , 40°C cell culture incubator or 40°C water bath, heating and culturing for 3h.
[0085] 3. Induction of apoptosis in hepatocellular carcinoma cells after heat shock
[0086] Move the hepatoma cells af...
Embodiment 3
[0091] Example 3: CD34-DC Tumor Vaccine Loaded with Apoptotic Heat Shock Breast Cancer Cell MCF7
[0092] 1. Heat shock treatment of breast cancer cell MCF7
[0093] Breast cancer cells MCF7 were inoculated in RPMI1640 cell culture medium with 10% FCS in 5% CO 2 , after 12 hours of incubation at 37°C in a cell incubator, the cells were moved to 5% CO 2 , 43°C cell culture incubator or 43°C water bath, heated for 4h.
[0094] 2. Induction of apoptosis in MCF7 breast cancer cells after heat shock
[0095] Return the MCF7 tumor cells after the above heat shock to 5% CO 2 After continuing to culture for 24 hours in a cell incubator at 37°C, the MCF7 tumor cells were collected. After 150GY γ-ray irradiation, in 5% CO 2 , 37°C cell incubator without serum for 36 hours, collected apoptotic MCF7 tumor cells, suspended in RPMI1640 medium.
[0096] 3. Isolation and purification of CD34 in vitro + Hematopoietic precursor cells, induced CD34-DCs (CD34 + Dendritic cells induced fro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com