Method of treating wastewater containing salt for producing hydrazine hydrate by carbamide method
A technology for salty wastewater and treatment methods, applied in the direction of oxidized water/sewage treatment, etc., can solve problems such as environmental pollution, resource waste, unusable salt water, etc., and achieve the effect of making full use of resources and avoiding waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
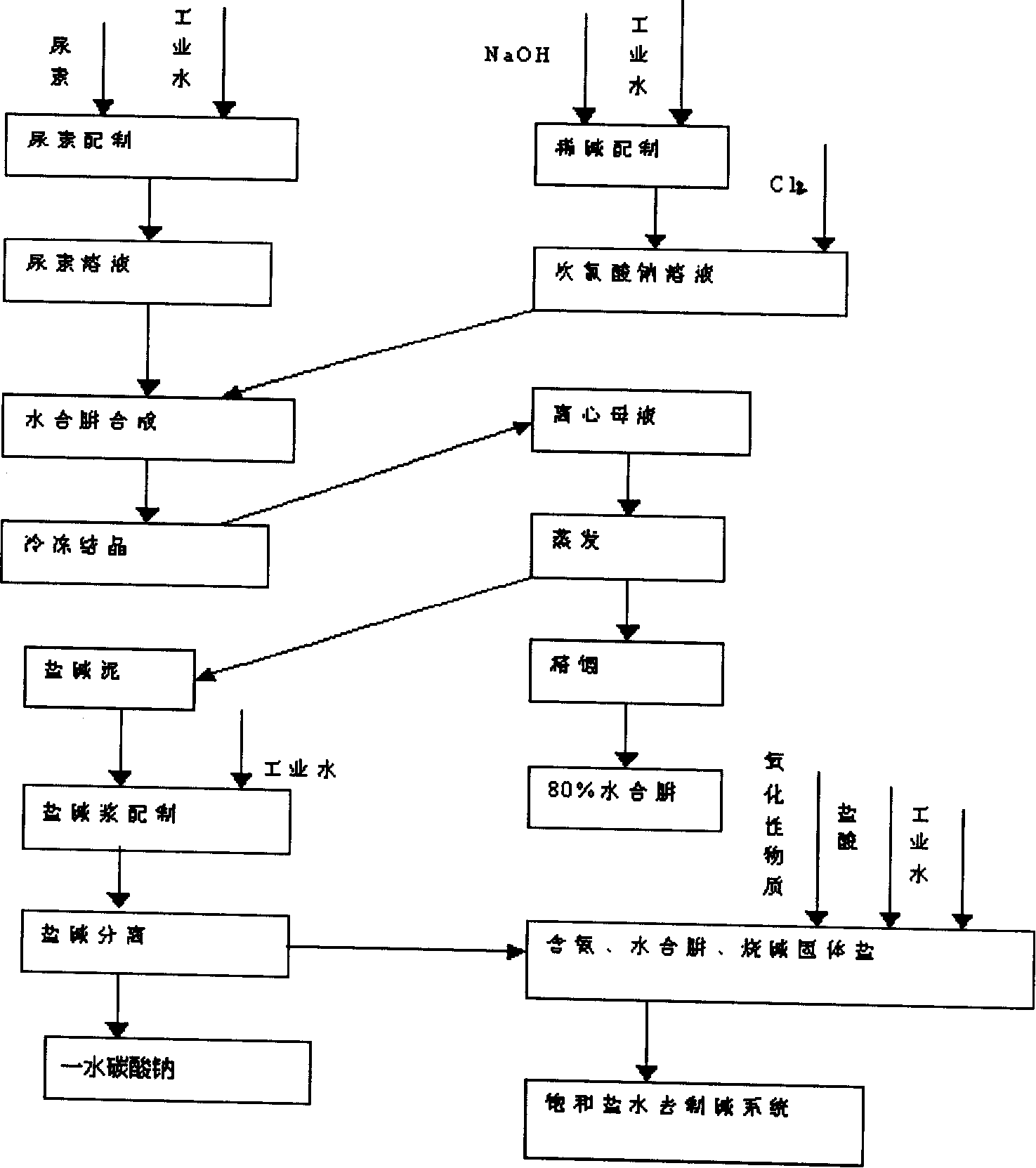
Image
Examples
Embodiment 1
[0034] Example 1: Using hydrogen peroxide as an oxidant to treat saline wastewater from the urea process.
[0035] Add the solid sodium chloride separated from saline-alkali recovery into the salt tank, control a certain height of the salt layer, and add the 280-300g / l brine prepared by adding industrial water into the brine storage tank. According to the NH contained in the brine, 3 with N 2 h 4 ·H 2 For the amount of O, add hydrogen peroxide of matching equivalent to it and use the pump to circulate the reaction. After the content of ammonia nitrogen is less than 15mg / l, according to the amount of NaOH contained in the brine, add hydrochloric acid of matching equivalent to it for circulation reaction. When the content of NaOH is less than 0.1 g / l, it is pumped to the caustic soda production system as raw brine. Among them, the following reaction takes place in the solution:
[0036]
[0037]
[0038]
Embodiment 2
[0039] Example 2: Using chlorine gas as an oxidant to treat saline wastewater from the urea process.
[0040] Add the solid sodium chloride separated from saline-alkali recovery into the salt tank, control a certain height of the salt layer, and add the 280-300g / l brine prepared by adding industrial water into the brine storage tank. According to the NH contained in the brine, 3 with N 2 h 4 ·H 2 For the amount of O, feed chlorine gas of matching equivalent into it and use the pump to circulate the reaction. After the content of ammonia nitrogen is less than 15mg / l, according to the amount of NaOH contained in the brine, add hydrochloric acid of matching equivalent to the inside for circulation reaction. When the content of NaOH is less than After 0.1g / l, it is pumped to the caustic soda production system as raw brine. Among them, the following reaction takes place in the solution:
[0041]
[0042]
[0043]
Embodiment 3
[0044] Example 3: Using sodium hypochlorite as an oxidant to treat saline wastewater from the urea process.
[0045] Add the solid sodium chloride separated from saline-alkali recovery into the salt tank, control a certain height of the salt layer, and add the 280-300g / l brine prepared by adding industrial water into the brine storage tank. According to the NH contained in the brine,3 with N 2 h 4 ·H 2 The amount of O, add matching equivalent sodium hypochlorite to it and use the pump to circulate the reaction. After the ammonia nitrogen content is less than 15mg / l, according to the amount of NaOH contained in the brine, add the matching equivalent hydrochloric acid to the inside for circulation reaction. When the NaOH content is less than 0.1 g / l, it is pumped to the caustic soda production system as raw brine. Among them, the following reaction takes place in the solution:
[0046]
[0047]
[0048] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com