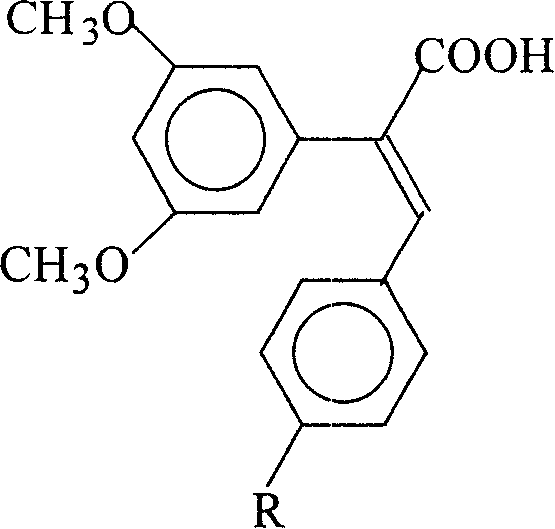
1-carboxy-1-(3,5-dimethoxy phenyl)-2-(4-r group phenyl) ethano and preparation method thereof
A dimethoxyphenyl and methoxyphenyl technology, which is applied in the preparation of carboxylate, nitrile, chemical instruments and methods, etc., can solve the problem of reducing cyclin protein expression, cell apoptosis, inability to complete, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of 1-carboxy-1-(3,5-dimethoxyphenyl)-2-(4-methoxyphenyl)ethene (3a)
[0034] 3, the preparation of 5-dimethoxyphenylacetonitrile (1):
[0035] Add 9.8 grams of sodium cyanide and 50 milliliters of water into a 250 milliliter three-necked flask, stir to dissolve the solid, add 50 milliliters of absolute ethanol, raise the temperature to 65°C, add 40 grams of 3,5-dimethoxybenzyl bromide, and The reaction was incubated for 1.5 hours. Pour out the reaction solution, cool to room temperature and precipitate a solid, filter to obtain a crude product, recrystallize from methanol / water to obtain 23 g of white needle-like crystals, melting point 51-53°C.
[0036] 3, the preparation of 5-dimethoxyphenylacetic acid (2):
[0037] In a 150 ml three-necked flask, 17.7 g of 3,5-dimethoxyphenylacetonitrile (1) and 50 ml of methanol were added, and the mixture was stirred and heated to dissolve the solid. Add 25 milliliters of water and 15 grams of sodium hydro...
Embodiment 2
[0040] Example 2: Preparation of 1-carboxy-1-(3,5-dimethoxyphenyl)-2-phenylethylene (3b)
[0041] Using a method similar to Example 1, 1.96 grams of 3,5-dimethoxyphenylacetic acid (2) was reacted with 1.1 grams of benzaldehyde to obtain white powder solid 1-carboxy-1-(3,5-dimethoxy Phenyl)-2-phenylethylene (3b) 1.7 g, melting point 203-205°C.
Embodiment 3
[0042] Example 3: Preparation of 1-carboxy-1-(3,5-dimethoxyphenyl)-2-(4-nitrophenyl)ethene (3c)
[0043] Using a method similar to Example 1, by reacting 1.96 grams of 3,5-dimethoxyphenylacetic acid (2) with 1.5 grams of 4-nitrobenzaldehyde, yellow needle-like crystals of 1-carboxy-1-(3,5 -2.2 g of -dimethoxyphenyl)-2-(4-nitrophenyl)ethylene (3c), melting point 226-228°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com