Modified release oral dosage form
A technology for oral dosage forms and dosage forms, applied in the field of solid oral dosage forms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0085] Nine batches of the lozenges of the present invention were prepared as follows.
[0086] 600Kg of isomalt powder type M was dissolved in 300Kg of water and steamed to 160°C, and then placed in a Hamac-Hansella candy processing machine under vacuum for 3 minutes to form a molten matrix. Then, when the bolted matrix is cooled to about 125°C, the nicotine ion exchange resin complex, xanthan gum, acesulfame potassium, sodium carbonate and flavoring agent are added and mixed. The mixture thus formed was transferred to the Ruffinatti kneading table for kneading. Then, the mixture is formed, rotated and sizing into a rope shape, and punched into lozenges with Bosch Uniplast.
[0087] The amount of lozenges per example *
[0088] Acesulfame potassium
[0089] * (The amount is calculated as "after processing" Kg)
Embodiment 10
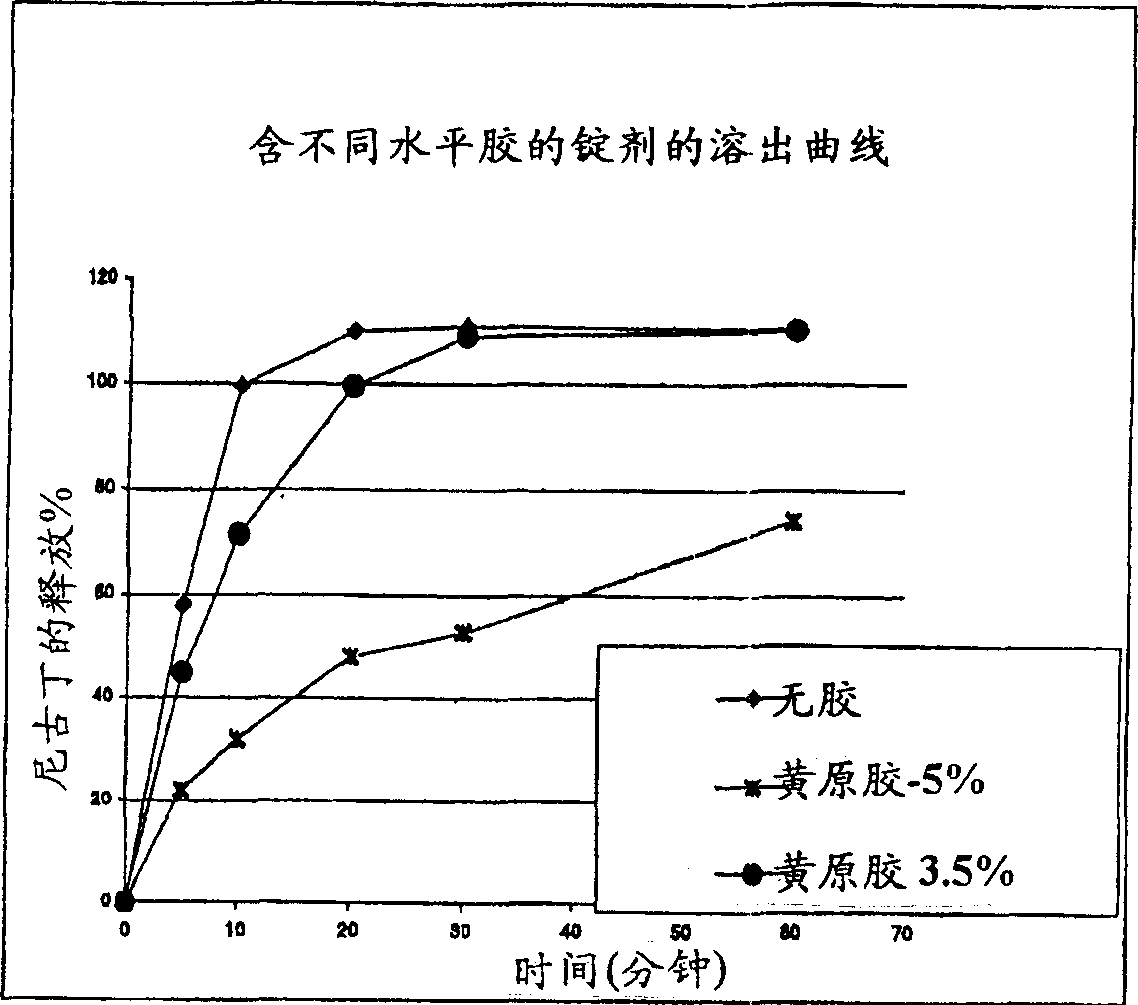
[0091] The measurement was performed according to the above Example 3 containing 3.5% xanthan gum nicotine tablets, according to Example 3 but containing 5.0% xanthan gum nicotine tablets, and the above-mentioned simultaneous pending PCT international application filed on March 22, 2002. The dissolution profile of the non-gelled gel formulation of No. PCT / US02 / 08914 (similar to Example 3 herein, but without gel).
[0092] The dissolution profile was determined by USP standard 3Bio.Dis., the tablet was immersed in a buffer solution of pH 7.4 at a standard rate to imitate the conditions in the oral cavity. figure 1 The graph in plots the measured percentage of nicotine release versus time (minutes).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com