CpG-N ODN gene sequence with high immunological activity CpG-S ODN and antagonism CpG-N ODN and use thereof
A technology of gene sequence and high immunity, applied in the direction of anti-infective drugs, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of no treatment methods and achieve the effect of high immune activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
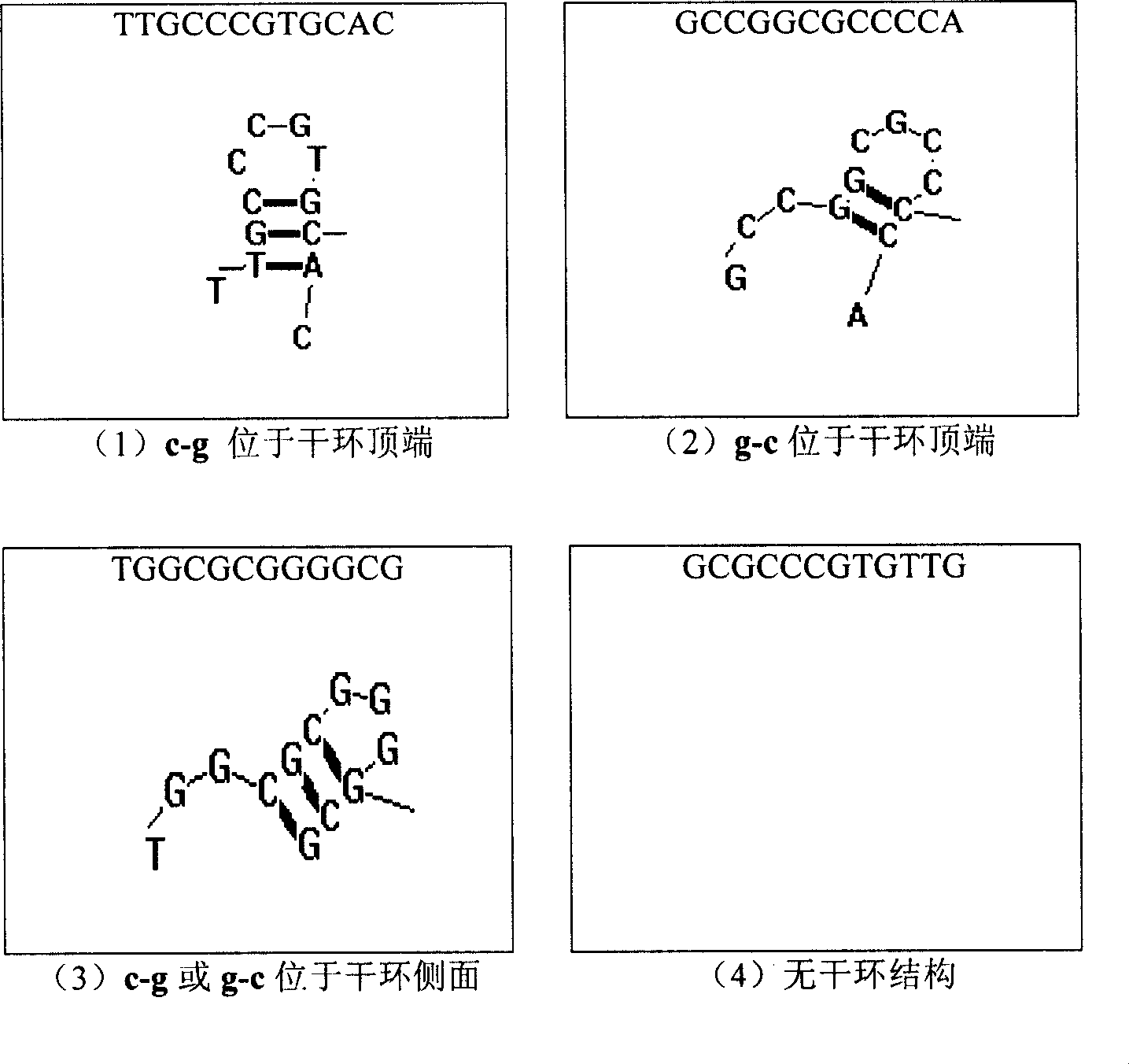
Image
Examples
experiment example 1
[0068] This experimental example is to obtain CpG-S ODN and CpG-N ODN gene sequences by using bioinformatics technology.
[0069] 1. The serotype 2, 5 and 12 Adv DNA and ECDNA sequences were obtained from the Nucleotide database on PubMed (ACCESSION: NC_001405, M73260, NC_001460, NC_000913).
[0070] 2. Using bioinformatics technology and using biological software such as Primer Premier 5 and BioEdit version 5.0.6, 256 CpG-containing hexanucleotide sequences in serotype 2, 5 and 12 Adv DNA and EC DNA were analyzed and compared (See Table 1), based on: ① High frequency in Adv DNA of serotype 2 and 5; ② Low frequency in Adv 12 DNA and EC DNA; ③ Adv2: Adv12≥2 or Adv2: EC≥5 In principle, 19 CpG-containing hexanucleotide sequences unique to serotype 2, 5 Adv DNA were identified.
[0071]
serial number
sequence
Adv 2
Adv 5
Adv 12
EC DNA
Adv2:Adv12
Adv2: EC
Frequency
frequency
Frequency
frequen...
experiment example 2
[0081] This experimental example is to study the synthesis method of oligonucleotides.
[0082] The method of the present invention adopts the solid-phase phosphoramidite triester method. Simply put, monomers in the solution form a 3'→5' phosphodiester bond through a condensation reaction, thereby connecting to a solid-phase support.
[0083] 1. Basic material:
[0084] (1) Support: Solid-phase synthesis is to immobilize nucleic acid on a solid-phase carrier to complete the synthesis reaction. The most commonly used solid-phase carrier is controllable microporous glass beads (CPG, controlled pore glass). The pore size of CPG depends on the synthesized The length of the oligonucleotide depends on the length of the oligonucleotide. Generally, when the synthetic chain length is less than 60nt, choose a pore size of 500 angstroms. Conditions for oligonucleotides up to 175nt. CPG is covalently bonded to the hydroxyl group of the initial nucleotide through a linker compound, and t...
experiment example 3
[0105] This example is to identify the activity of 7 non-obviously irritating ODNs inhibiting CpG-S ODN.
[0106] Isolate and culture human peripheral blood mononuclear cells (hPBMC), adjust the concentration of hPBMC cell suspension to 1×10 6 / ml, added to a 24-well plate, 1ml per well. Set at 37°C CO 2 After culturing in the incubator for 2 hours, add 10 μg / ml of each ODN to be screened for 30 minutes, then add 5 μg / ml of CpG-S ODN (TGG CGC GGGGCG), and place at 37°C CO 2 The cell culture supernatant was taken after 4 hours of incubation in the incubator, and the concentration of TNF-α in the cell culture supernatant was determined by double antibody sandwich ELISA method to clarify the ability of the above 7 ODNs to inhibit the release of TNF-α from hPBMC induced by CpG-S ODN. The experimental results showed that the above 7 ODNs all had the ability to inhibit the release of TNF-α from hPBMC induced by CpG-S ODN, so we officially called it CpG-N ODN (see Table 5).
[010...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com