Melittin and use thereof
A technology of melittin and endotoxin, applied in the fields of peptides, antibacterial drugs, drug combinations, etc., can solve problems such as inaccurate curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the synthesis of melittin
[0037]1.1 Synthesis of melittin resin peptide resin: Weigh 0.05mmol of Fmoc-L-Lys-PEG-PS resin (or other polypeptide synthetic resin) and pour it into a synthesis column, add about 15ml of refined dimethylformamide (DMF) to swell 30min, take another 50ml of 1.0mmol diisopropylethylamine (DIPEA) (8.7ml DIPEA+41.3ml DMF), 0.5mmol of azabenzotriazole methyluronium hexafluorophosphate (HATM) 50ml (9.5g HATM+40.5 ml DMF) and an appropriate amount of DMF (Deblock solution) containing 20% hexahydropyridine were added to the reagent bottle respectively, and 2 mmol of Fmoc-amino acids were successively weighed in a suitable container according to the melittin amino acid sequence of the present invention. According to the solid-phase chemical synthesis method invented by merrifield, the amino acid sequence of melittin is sequentially carried out from the C-terminus. Add the Deblock liquid depeptidation resin Fmoc protecting group to the...
Embodiment 2
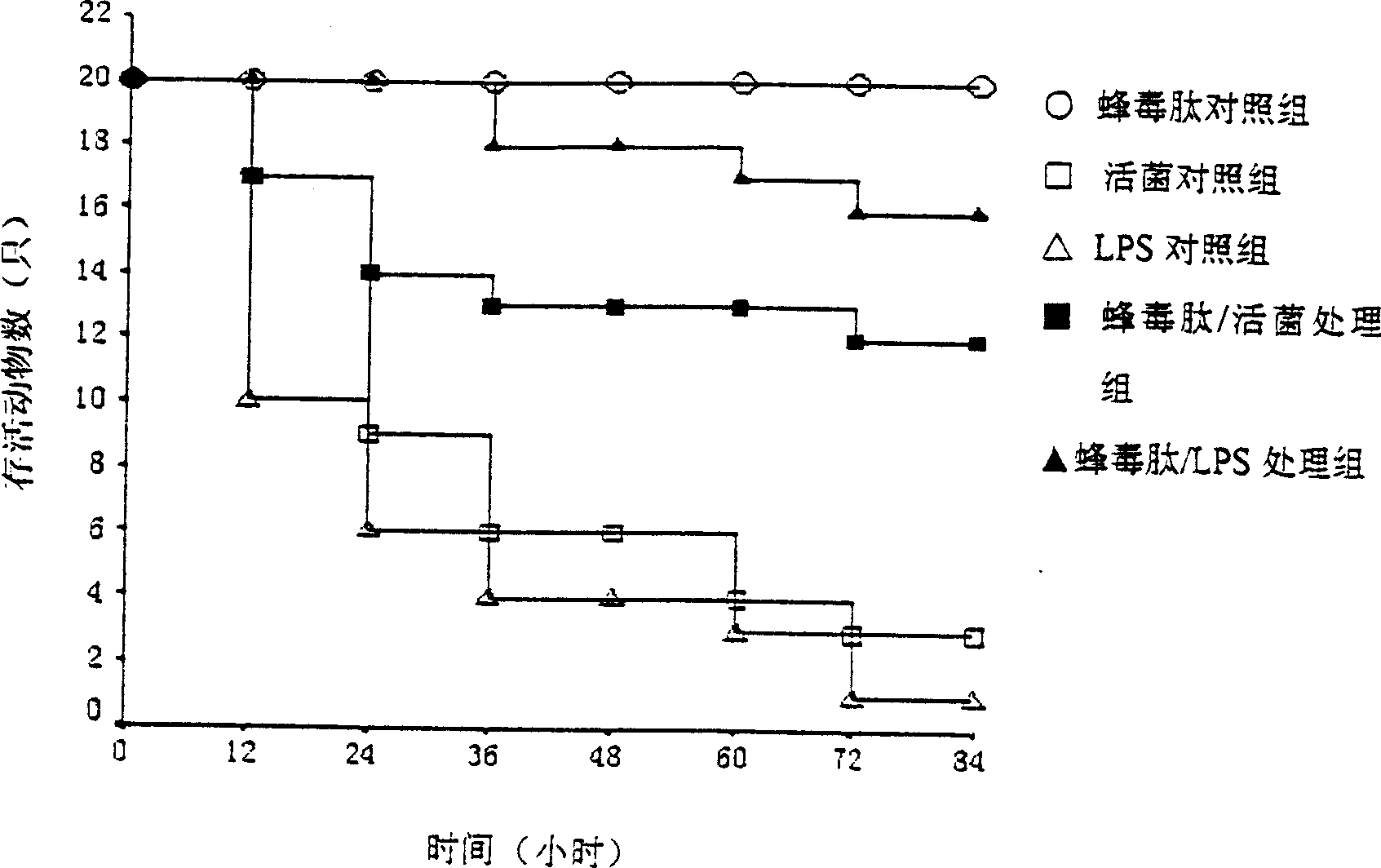
[0047] Example 2: Protective effect of melittin on Escherichia coli ATCC25922 live bacteria / LPS challenged mice
[0048] 2.1 Experimental method: Kunming mice were randomly divided into 5 groups, 20 in each group, melittin control group, live bacteria control group and LPS control group were injected with melittin (3 mg / kg), E. ATCC25922 (2×10 9 CFU / 20g body weight) and LPS 20mg / kg; 9 CFU / 20g body weight) were injected with melittin (3mg / kg) within 20s, and the melittin / LPS treatment group was injected with LPS 20mg / kg 10 seconds after the injection of melittin 3mg / kg. The total volume injected was 200 μl / 20 g body weight. The mice were observed for death within 3 days.
[0049] 2.2 Experimental results: The mental state of the mice in the melittin treatment group was sluggish but recovered quickly, and their appetite, activity and responsiveness to external stimuli were improved in a short period of time, which were better than those in the live bacteria / LPS control group....
Embodiment 3
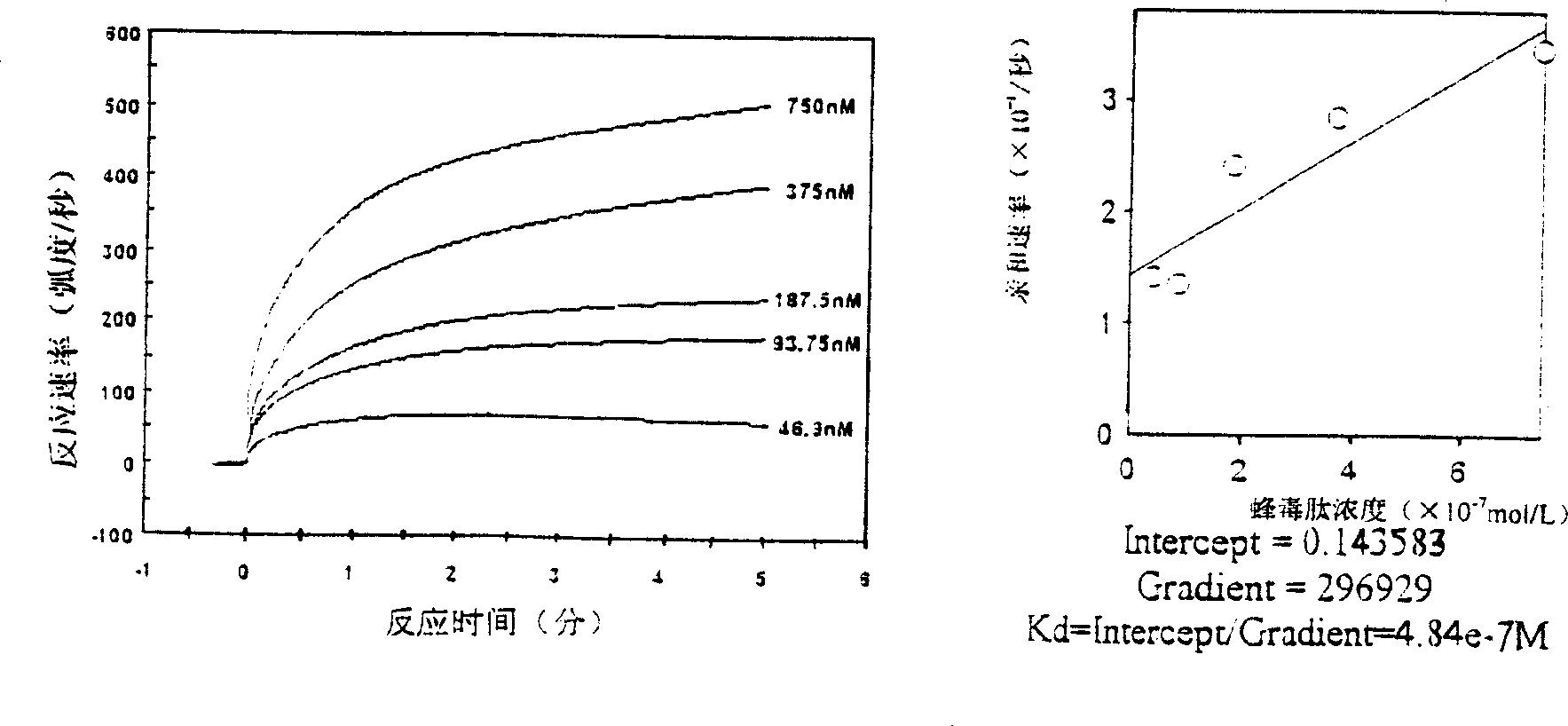
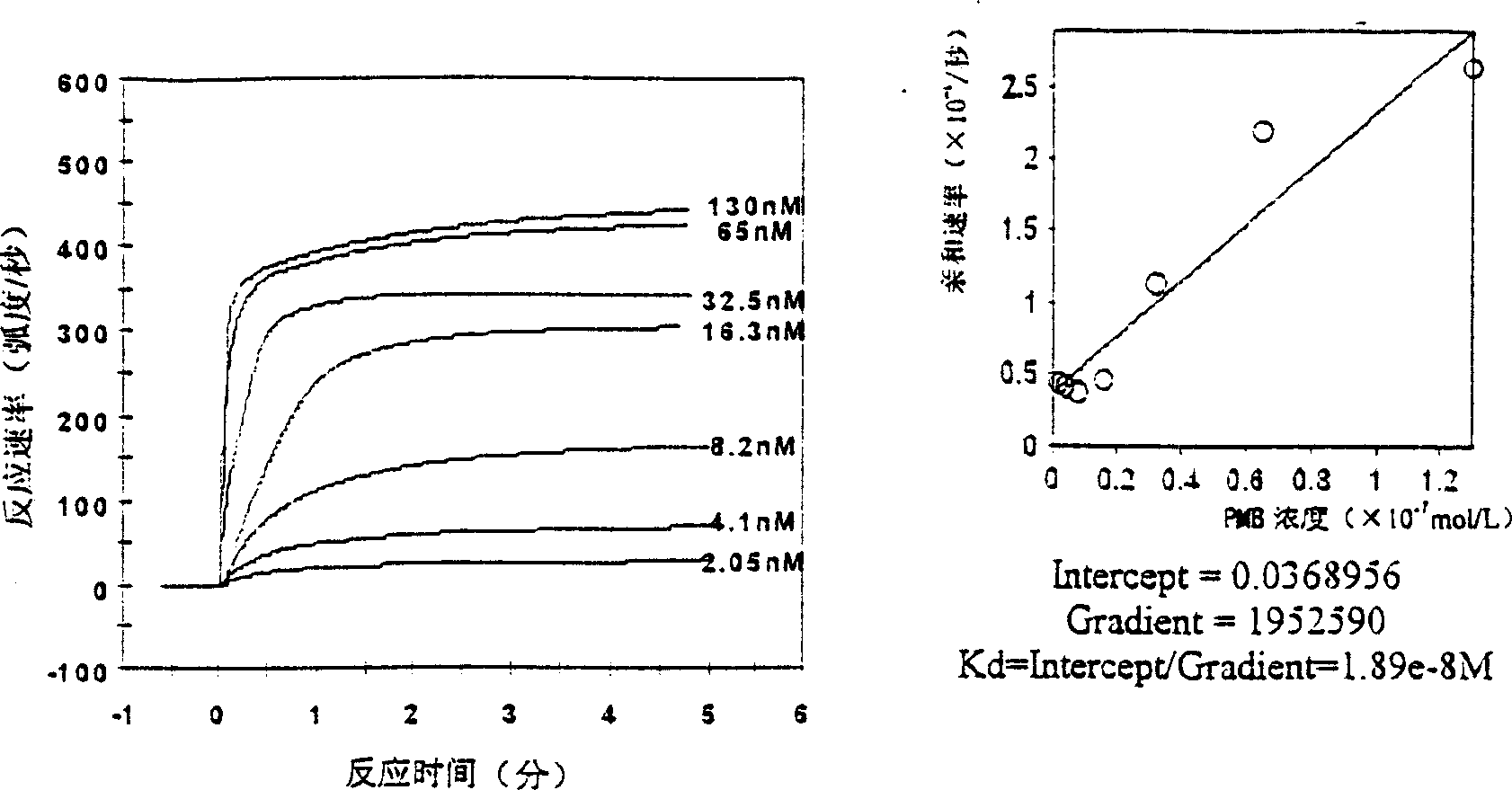
[0050] Embodiment 3: the antibacterial action of melittin
[0051] 3.1 Experimental method: The antibacterial activity of melittin against 18 strains of bacteria was detected by microdilution method and compared with other antibiotics. ① Take Escherichia coli ATCC25922 and inoculate it in LB culture medium, incubate at 37°C for 18 hours, then transfer to fresh culture medium and cultivate it for 4 hours, and use LB culture medium to adjust the bacterial concentration to 1×10 5 CFU / ml. ②Take a 96-well culture plate, with 8 wells as a column, a total of 12 columns. Add 200 μl of bacterial solution to the first column, add 100 μl of bacterial solution to the second to eleventh columns, and add 100 μl of LB culture solution to the last column as a negative control. ③Take melittin and 6 kinds of other antibiotics, each diluted with normal saline to 10.24mg / ml. ④ The first line is for doubling dilution of melittin. Add 10 μl of melittin to the first well, mix well, aspirate 100 μ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com