Geen light emitting vycor glass production method
A technology of high-silica glass and its manufacturing method, applied in the field of glass, can solve problems such as unrealized breakthroughs with practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
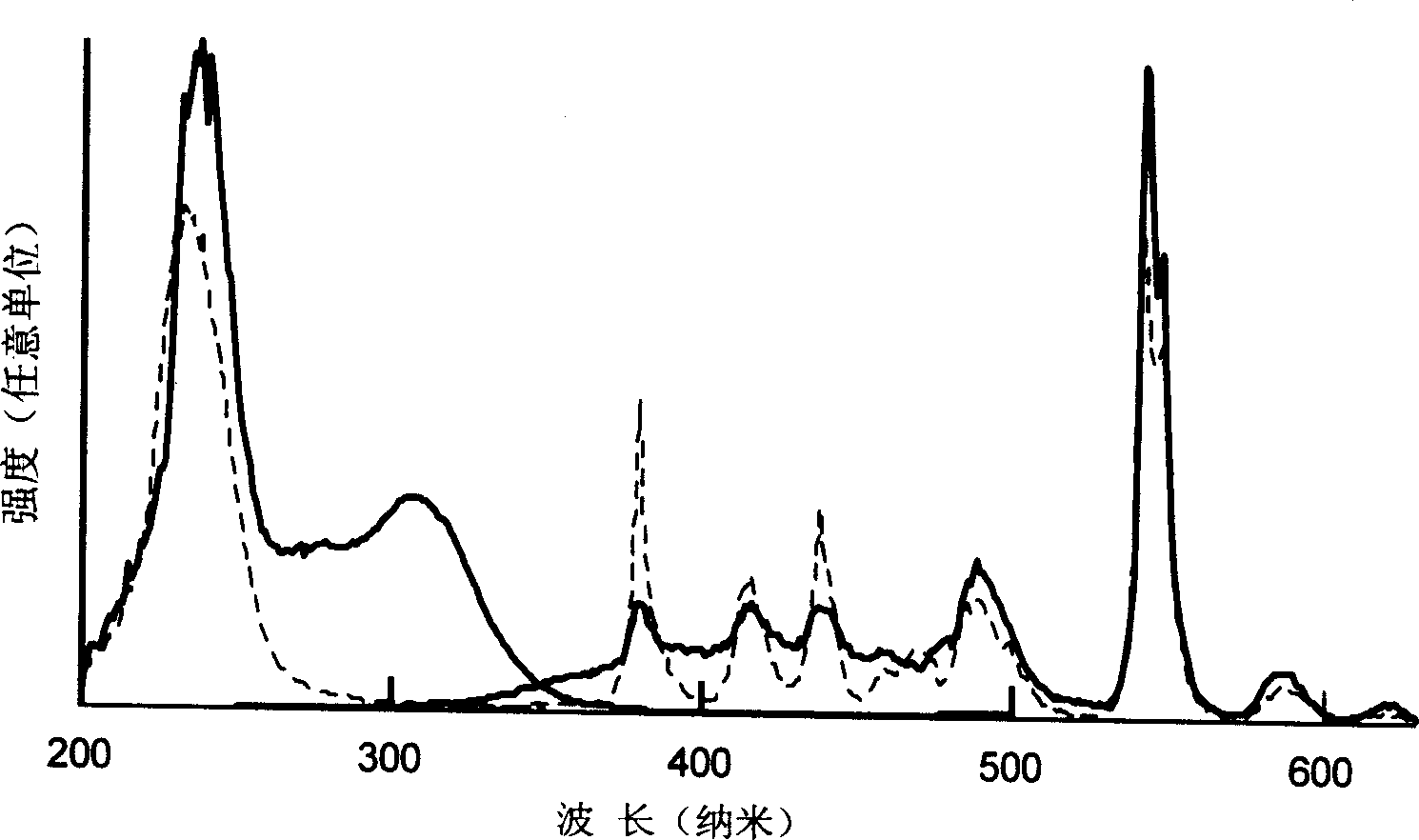
[0019] Will be decomposed equivalent to 0.4g of Tb 2 o 3 1.12g of analytically pure Tb(NO 3 ) 3 9H 2 O was put into 25 ml of ethanol and acetone solution, and after it was completely dissolved, the SiO 2 The porous glass with a content of more than 97wt% is put into the solution and soaked for more than 10 minutes; after that, the high-silica microporous glass doped with terbium ions is put into a high-temperature furnace, and undergoes solid-phase sintering at 1150°C to eliminate micropores and become Dense and transparent Tb-doped 2 o 3 High silica glass with a concentration of about 0.4%. During the sintering process, after rising from room temperature to 400°C at a speed of less than 5°C per minute, it rises to around 950°C at a speed of 10°C per minute, and then rises from this temperature at a speed of below 5°C per minute. After reaching 1150°C and keeping the temperature at this temperature for more than 30 minutes, turn off the power supply of the high-temperat...
Embodiment 2
[0021] Decompose Tb equivalent to 0.1~1.2g 2 o 3 0.28~3.36g of analytically pure Tb(NO 3 ) 3 9H 2 O is put into 25 ml of hydrochloric acid solution (or nitric acid and sulfuric acid) with a concentration of 0.5 to 3 moles, and after it is completely dissolved, the SiO 2 The porous glass with a content of more than 95wt% is put into the solution and soaked for more than 10 minutes; after that, the high-silica porous glass doped with terbium ions is put into a high-temperature furnace, and undergoes solid-state sintering at 1050-1200°C to eliminate micro Pores become dense and transparent Tb-doped 2 o 3 High silica glass with a concentration of about 0.1 to 1.2%. During the sintering process, after rising from room temperature to 400°C at a speed of less than 5°C per minute, it rises to around 950°C at a speed of 10°C per minute, and then rises from this temperature at a speed of below 5°C per minute. After reaching 1050-1200°C and keeping the temperature at this temperat...
Embodiment 3
[0023] Will be decomposed equivalent to 0.05g of Tb 2 o 3 0.14g of analytically pure Tb(NO 3 ) 3 9H 2 O and decomposed Gd equivalent to 0.1g 2 o 3 0.3g of analytically pure Gd(NO 3 ) 3 9H 2 O, equivalent to 0.02g of Y after decomposition 2 o 3 0.07g of analytically pure Y (NO 3 ) 3 ·6H 2 O and equivalent to 0.05g Ce after decomposition 2 o 3 0.13g of analytically pure Ce(NO 3 ) 3 ·6H 2 O is put into 25 ml of 1.0 molar concentration of nitric acid solution or aqueous solution, after it is completely dissolved, and then the size is 5 × 5 × 3mm, SiO 2 The porous glass with a content of more than 96% (wt%) is put into the solution and soaked for more than 10 minutes; after that, the high-silica microporous glass doped with these ions is put into a high-temperature furnace, and undergoes solid-state sintering at 1120°C , Eliminate micropores and become dense and transparent high-silica glass with a total concentration of about 0.22% doped with various oxides. Dur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com