Oligomeric compounds for the modulation of survivin expression
A compound and analog technology, applied in the field of oligomeric compounds, can solve problems such as limiting the commercial value of analogs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0235] Embodiment 1: monomer synthesis
[0236] LNA monomer modules and their derivatives were constructed according to the following published procedures and references cited here, see:
[0237] ●WO 03 / 095467A1;
[0238] D. S. Pedersen, C. Rosenbohm, T. Koch (2002) Preparation of LNA Phosphoramidites, Synthesis 6, 802-808;
[0239] ●M.D.Sφensen, L.Kvaernφ, T.Bryid, A.E.Hakansson, B.Verbeure, G.Gaubert, P.Herdewijn, J.Wengel (2002) α-L-ribo-configured Locked Nucleic Acid (o-l-LNA): Synthesis and Properties, J. Am. Chem. Soc., 124, 2164-2176;
[0240] S.K.Singh, R.Kumar, J.Wengel (1998) Synthesis of Novel Bicyclo[2.2.1] Ribonucleosides: 2′-Amino-and 2′-Thio-LNA Monomeric Nucleosides, J.Org.Chem.1998, 63, 6078 -6079;
[0241]C. Rosenbohm, S.M.Christensen, M.D.Srensen, D.S.Pedersen, L.E.Larsen, J.Wengel, T.Koch (2003) Synthesis of 2'-amino-LNA: a newstrategy, Org.Biomol.Chem.1, 655-663 .
[0242] Synthesis of 2'-thio-LNA ribothymidine phosphoramidite. Reagents and Conditio...
Embodiment 2
[0288] Embodiment 2 synthetic oligonucleotides
[0289] Oligonucleotides were synthesized at 1 or 15 μmol using the phosphonimide method on an Expedite 8900 / MOSS synthesizer (Multiple Oligonucleotide Synthesis System, multi-step oligonucleotide synthesis system). At the end of the synthesis (DMT-on), oligonucleotides were cleaved from the solid support by using ammonia for 1 hour at room temperature and deprotected for 3 hours at 65°C. Oligonucleotides were purified by reverse phase HPLC (RP-HPLC). After removing the DMT group, the oligonucleotides were identified by IE-HPLC or RP-HPLC. Oligonucleotide identities were identified by ESI-MS. It will be described in detail below.
[0290] Preparation of LNA succinyl half ester
[0291] 5'-O-Dmt-3'-hydroxy-LNA monomer (500 mg), succinic anhydride (1.2 equiv) and dimethylaminopyridine (1.2 equiv) were dissolved in DCM (35 mL). The reaction was carried out overnight with shaking at room temperature. with NaH 2 PO 4 0.1M, pH5...
Embodiment 3
[0319] Example 3: Detection of designed oligomeric compounds
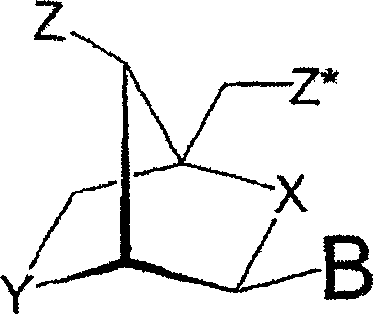
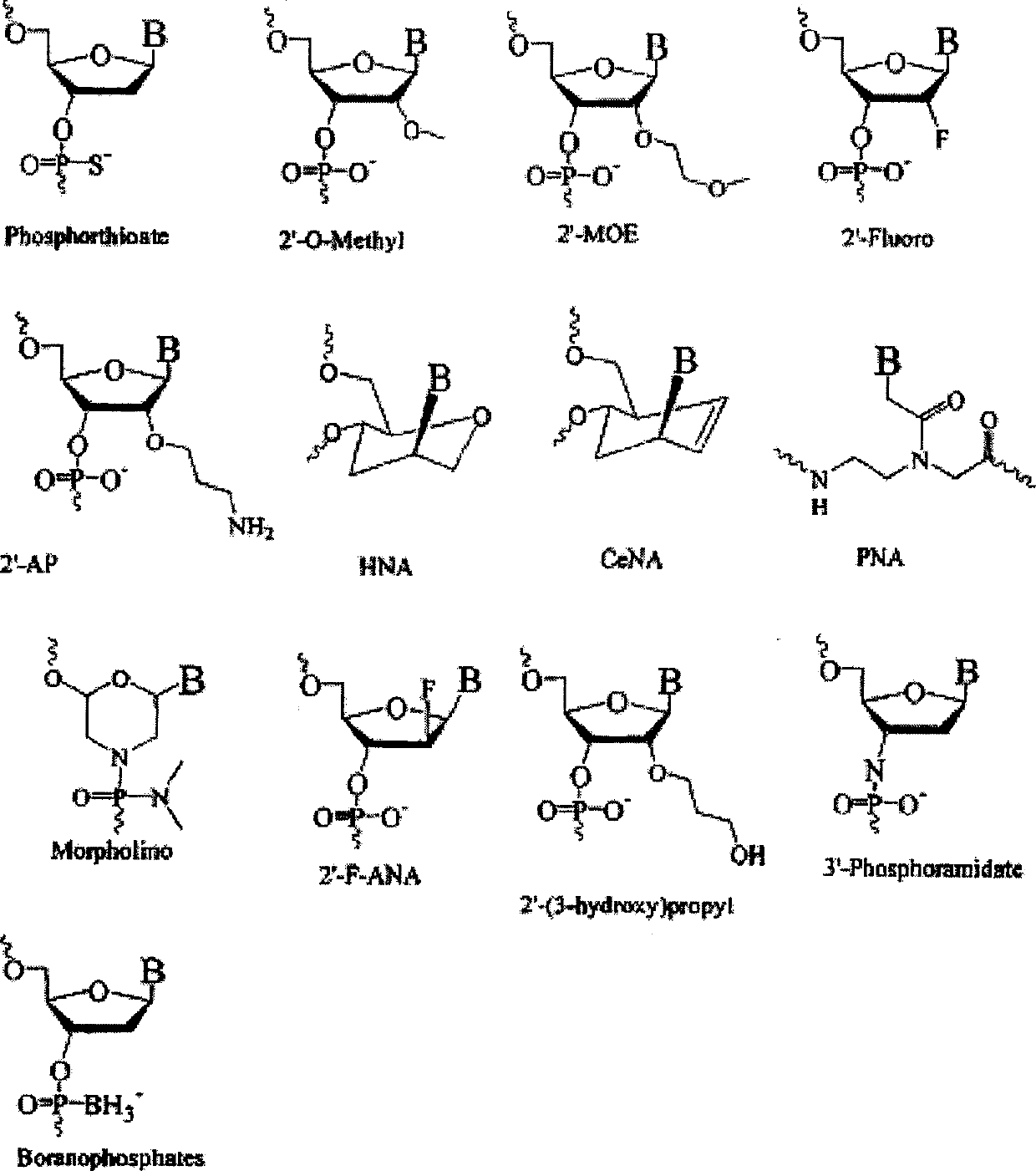
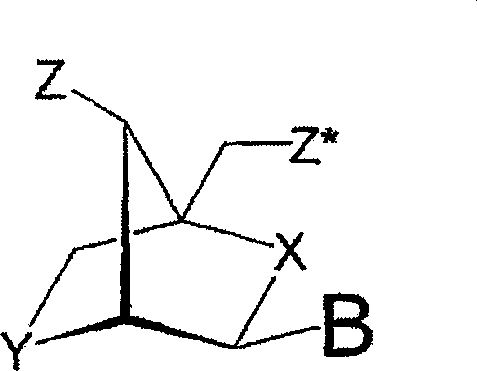
[0320] Our interest was to evaluate the antisense activity of different designs of oligonucleotides to demonstrate the importance of choosing the best design of nucleotides targeting survivin. For this purpose, we devised an in vitro assay that allowed us to screen different oligonucleotide designs by measuring the activity of firefly (Photinus pyralis) luciferase after downregulation with antisense oligonucleotides. Figure 1 contains an illustration of most of the designs mentioned in this paper. In the initial screen, designs containing β-D-oxy-LNA were evaluated, all of which targeted the motifs within the mRNAs being tested. Designs consisting of interpolymers with different gap sizes, different numbers of phosphorothioate internucleoside linkages, and different numbers of LNAs were examined. Headers and intermers with different numbers of β-D-oxy-LNA, different numbers of phosphorothioate internucleoside lin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com