An assembly for the preparation of a medical device having a coating comprising hydrogen peroxide
A hydrogen peroxide, medical device technology, applied in the field of hydrophilic coating, medical use, can solve the problem of hydrogen peroxide stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0205] Example 1 - Preparation of Catheter Coated with Polyvinylpyrrolidone
[0206]A urinary catheter with a hydrophilic coating of polyvinylpyrrolidone can be prepared as follows:
[0207] a) Prepare first and second solutions of high molecular weight polyvinylpyrrolidone (PVP) (for example, Plasdone K-90) dissolved in N-methylpyrrolidone (NMP), ethanol and Citrofol A1 including photoinitiator in solvent / plasticizer mixtures. The PVP content of the solution is in the range of 1-8% (w / w). The first and second solutions may be the same.
[0208] b) Soak the polyurethane raw catheter in the first solution and let it dry at room temperature for 10-120 seconds.
[0209] c) Soak the resulting catheter in a second solution of PVP.
[0210] d) Further drying the catheter at higher temperature (eg, at 70-80°C).
[0211] e) crosslinking the PVP by exposing the coated conduit to UV light in the wavelength range of 200nm-300nm for 1 / 2-15 minutes.
[0212] f) Place the cross-linked...
Embodiment 2
[0214] Example 2 - Preparation of Catheters Comprising Hydrogen Peroxide
[0215] The preparation of a sterile catheter with a hydrophilic coating comprises the following steps:
[0216] a) Prepare first and second solutions of high molecular weight polyvinylpyrrolidone (PVP) (for example, Plasdone K-90) dissolved in N-methylpyrrolidone (NMP), ethanol and Citrofol A1 including photoinitiator in solvent / plasticizer mixtures. The PVP content of the solution is in the range of 1-8% (w / w). The first and second solutions may be the same.
[0217] b) Soak the polyurethane raw catheter in the first solution and let it dry at room temperature for 10-120 seconds.
[0218] c) Soak the resulting catheter in a second solution of PVP.
[0219] d) Further drying the catheter at higher temperature (eg, at 70-80°C).
[0220] e) crosslinking the PVP by exposing the coated conduit to UV light in the wavelength range of 200nm-300nm for 1 / 2-15 minutes.
[0221] f1) Place the cross-linked co...
Embodiment 3
[0226] Example 3 - Preparation of Catheter Assembly
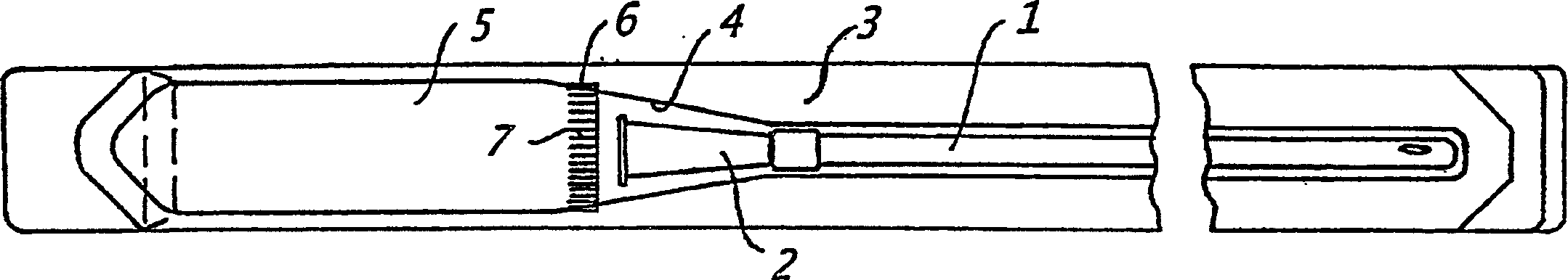
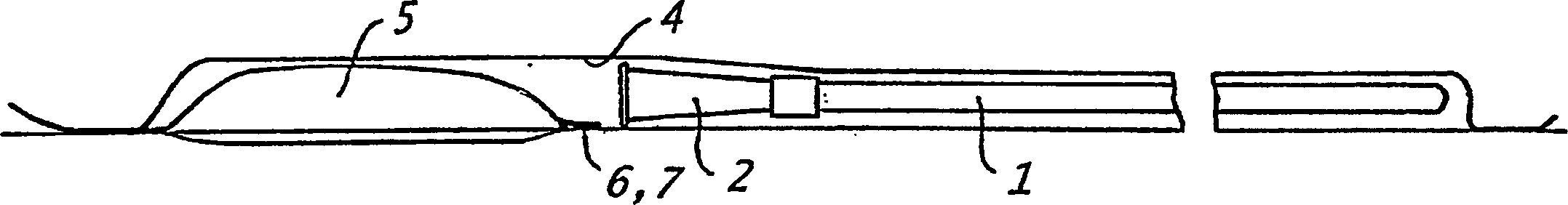
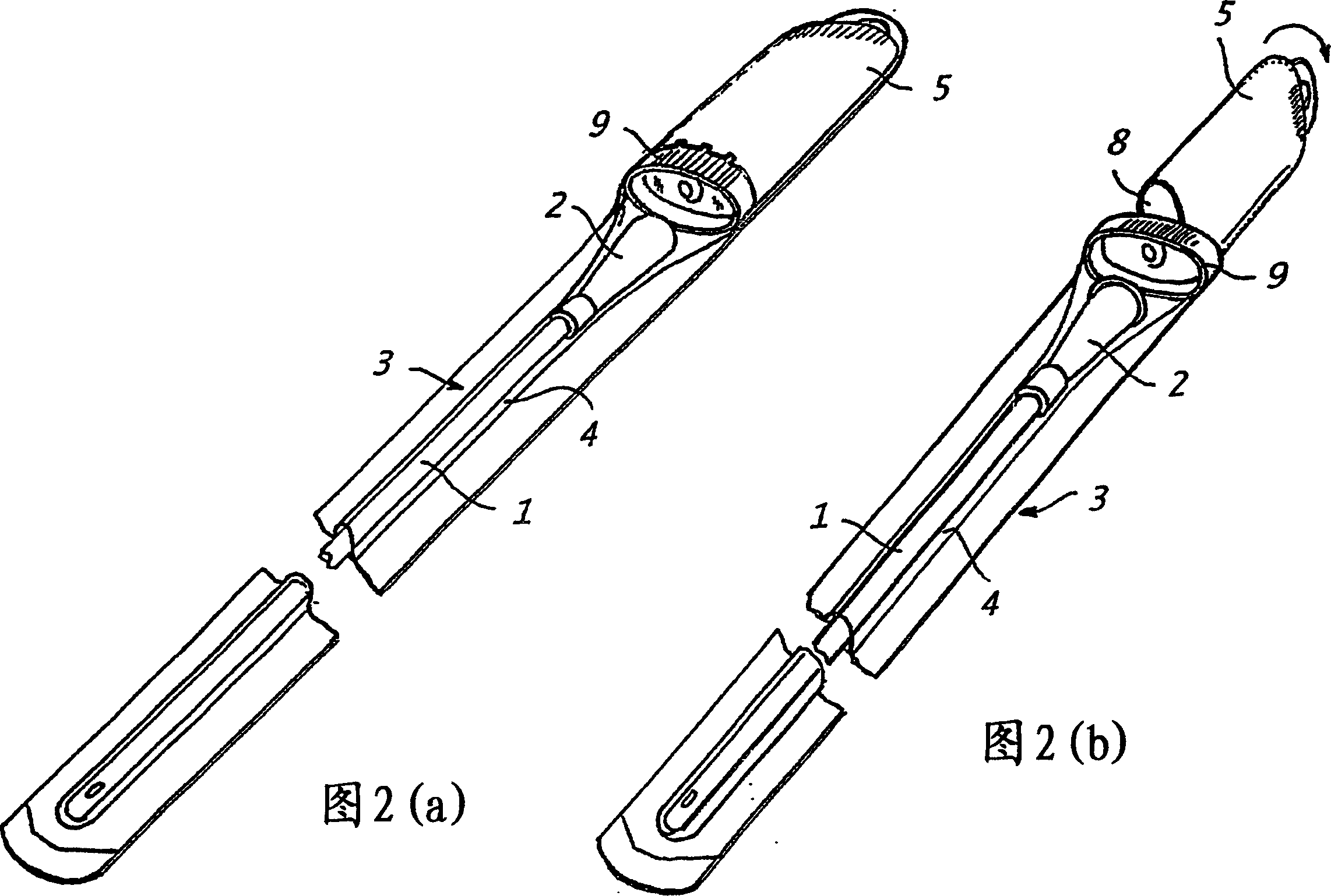
[0227] The catheter assembly shown in Figure 2 can be prepared as follows:
[0228] A dry urinary catheter (1) coated with polyvinylpyrrolidone (PVP) was prepared as described in Example 1. The catheter is placed in the first chamber (4) of the packaging device (3), which chamber is sealed by welding. Prepare a formulation containing aqueous hydrogen peroxide, DETAPMP, and an osmolarity-enhancing formulation (e.g., Na 2 SO 4 or NaNO 3 ) of liquid expansion medium (for example, as shown in Example 2) and loaded into the second chamber formed by the outer rigid container (5), which is installed as an integral part of the packaging device (3) . As shown in Figure 2 (b), the container (5) is installed to be able to rotate about 90 degrees relative to the end wall (8), so that the end wall (8) of the container (5) is provided towards the chamber (4) The liquid outlet and inlet of the rigid end wall (9) of the rigid end wal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com