Self-catalytic reduction ammonia-leaching method for multi-metal concretion in deep sea
A polymetallic nodule and self-catalysis technology, applied in the direction of improving process efficiency, can solve the problems of increased carbon monoxide consumption, large leaching equipment, complex process, etc., and achieve the effect of improving processing capacity and reducing carbon monoxide consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
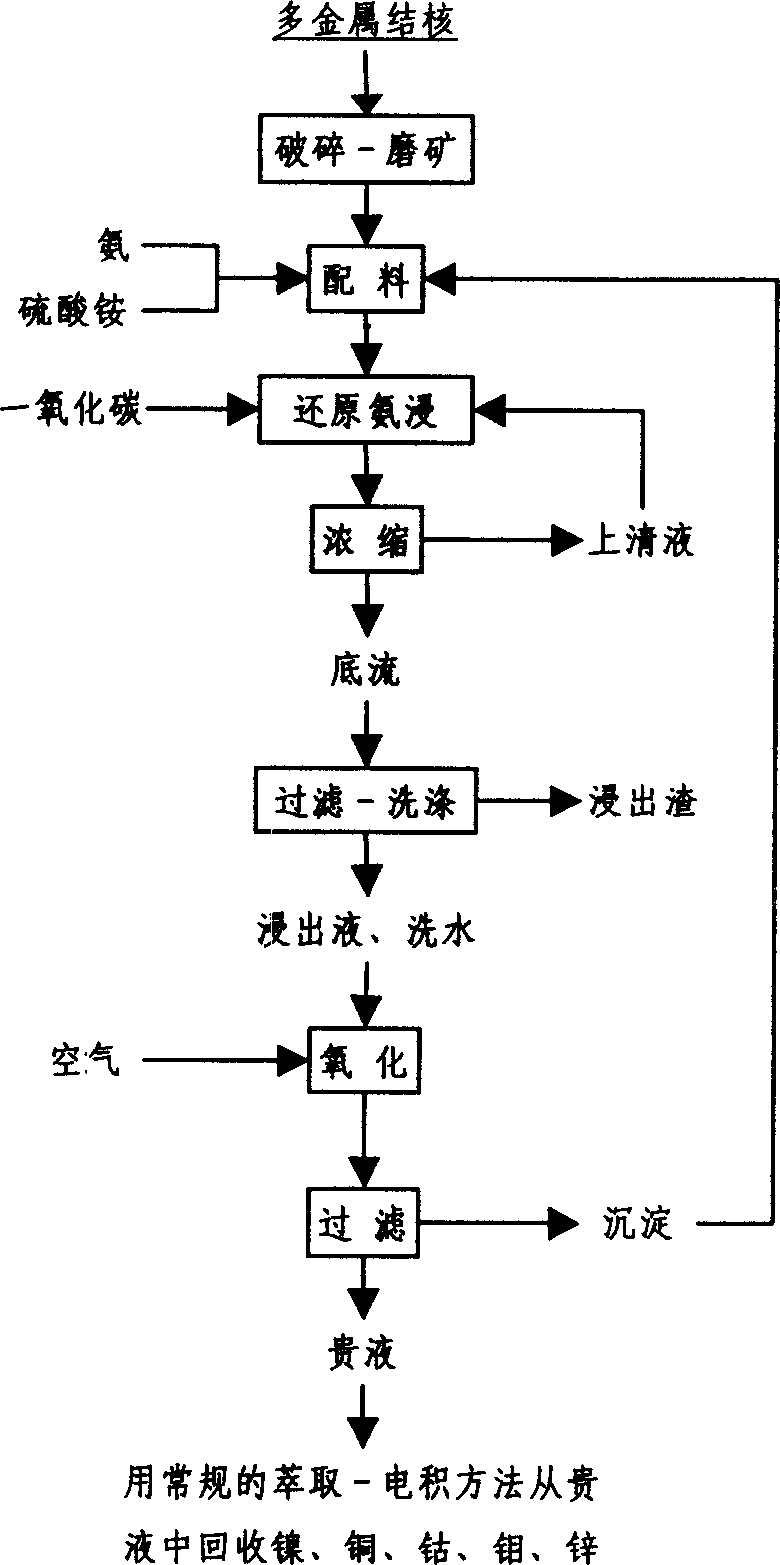
Image
Examples
Embodiment 1
[0043] Take 10g of rough polymetallic nodules, grind them to -0.074mm, accounting for 84.76%, add to 120g / l NH 3 , 40g / l SO 4 2- , 2.5g / l Co 2+ , 8.6g / l Ni 2+ , 15.5g / l Cu + In the ammonia-ammonia sulfate solution, the liquid-solid weight ratio is 40:1, the leaching temperature is 40°C, and the carbon monoxide flow rate is 1m 3 / t·min, leaching for 2h. The leaching rate of cobalt is 91.21%.
Embodiment 2
[0045] Take 10g of rough polymetallic nodules, grind to -0.074mm to account for 70%, add to 160g / l NH 3 , 100g / l SO 4 2- , 2.5g / l Co 2+ , 8.6g / l Ni 2+ , 20g / l Cu + In the ammonia-ammonia sulfate solution, the liquid-solid weight ratio is 20:1, the leaching temperature is 50°C, and the carbon dioxide flow rate is 2m 3 / t·min, leaching for 0.5h. The leaching rate of cobalt is 92.9%.
Embodiment 3
[0047] Take 10g of rough polymetallic nodules, grind to -0.074mm to account for 50%, add to 100g / l NH 3 , 200g / l SO 4 2- , 1.9g / l Co 2+ , 11.3g / l Ni 2+ , 8g / l Cu + In the ammonia-ammonia sulfate solution, the liquid-solid weight ratio is 5:1, the leaching temperature is 55°C, and the carbon monoxide flow rate is 0.4m 3 / t·min, leaching for 2h. The leaching rate of cobalt is 89.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com