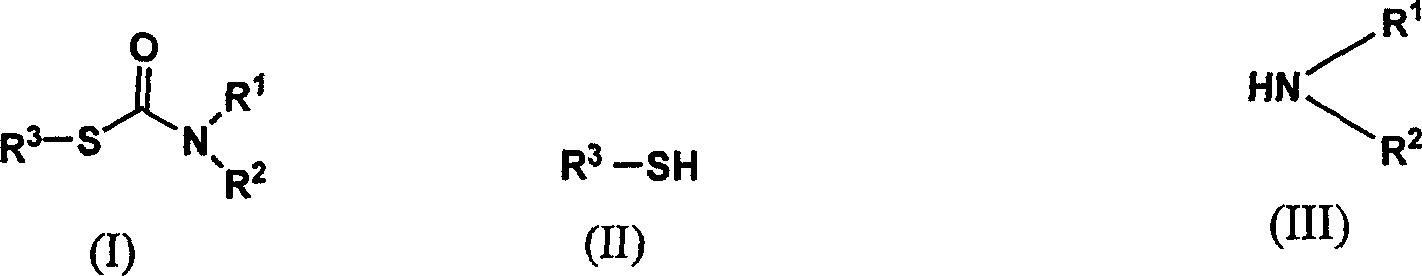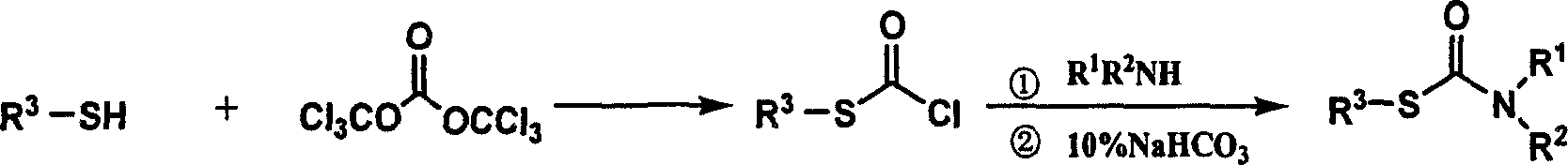Method of synthesizing thiocarbamate by 'one pot method'
A technology for synthesizing thiocarbamate and carbamate, applied in organic chemistry, etc., can solve the problems of expensive catalyst, harsh reaction conditions, difficult post-processing, etc., and achieve great potential social and economic benefits, mild reaction conditions, Easy to store for easy access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The molar ratio of the feed material is substituted mercaptan: bis(trichloromethyl)carbonate: amine is 1.0:0.33:1.0, wherein the substituted mercaptan is p-cresol, the amine compound is aniline, and the organic solvent is methylene chloride , and its dosage is 15 times of the quality of p-cresol.
[0017] In a 150 mL four-neck flask equipped with a thermometer, a reflux condenser, and mechanical stirring, 40 mmol of p-cresylthiophenol, 13.3 mmol of bis(trichloromethyl)carbonate and 74.4 g of dichloromethane were added. Start stirring, dissolve, and react at room temperature for 1 hour. TLC traces until the reaction of the raw materials is complete. Cool the reaction solution to 0-5°C in an ice bath. Raise the temperature to reflux, stir for 2 h, cool to room temperature after the reaction, and wash the reaction solution with 10% NaHCO3, H2O, and saturated brine to obtain an aqueous phase and an organic phase, discard the aqueous phase, and dry the organic layer with an ...
Embodiment 2
[0019] The molar ratio of the feed material is substituted mercaptan: bis(trichloromethyl)carbonate: amine is 1.0:0.40:1.0, wherein the substituted mercaptan is p-cresol, the amine compound is aniline, and the organic solvent is methylene chloride , and its dosage is 15 times of the quality of p-cresol.
[0020] Others are with embodiment 1. The yield of the obtained thiocarbamate product is 78.8%, the melting point of the product is 129.0-130.0° C., and the purity is 98.5%.
Embodiment 3
[0022] The molar ratio of the feed material is substituted thiol: bis(trichloromethyl)carbonate: amine is 1.0:0.50:1.0, wherein the substituted mercaptan is p-cresol, the amine compound is aniline, and the organic solvent is methylene chloride , and its dosage is 20 times the mass of p-cresol.
[0023] Others are with embodiment 1. The yield of the obtained thiocarbamate product is 80.3%, the melting point of the product is 129.2-130.1° C., and the purity is 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com