Process for prepering active magnesium oxide
A technology of activated magnesia and light-burned magnesia, which is applied in the field of inorganic chemical industry, can solve the problems of raw material origin limitation, energy consumption and high production cost, and achieve the effects of low production cost, easy operation and reduced environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
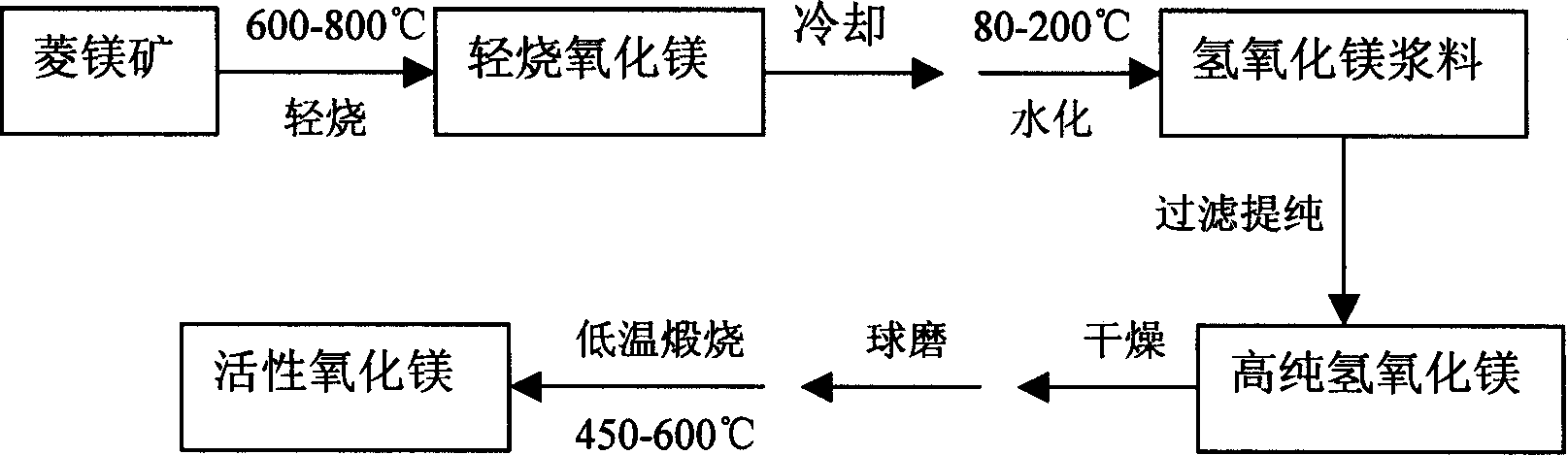
[0027] 1. Light burning. Add magnesite (MgO content is 44.0% by weight) into a roasting furnace, and roast at 800° C. for 60 minutes to obtain light-burned magnesium oxide.
[0028] 2. Cool down. Naturally cool the light-burned magnesia to 200°C in air.
[0029] 3. Hydration. Put the cooled light-burned magnesia into water 5 times the volume of the mineral, mix, add the mixed slurry into the ball mill tank, and ball mill for 40 minutes, then heat the ball-milled slurry to 160°C, Rapid hydration of MgO under temperature conditions to generate Mg(OH) 2 .
[0030] 4. Purification. Purify by filtering, keep the slurry and discard the filter residue.
[0031] 5. Dry. The slurry was dried at 160° C. for 10 hours to remove moisture.
[0032] 6. Ball milling. The dried Mg(OH) 2 Place in a ball mill jar and grind to -325 mesh.
[0033] 7. Calcining at low temperature. Mg(OH) after low temperature calcination and ball milling 2 , the calcination temperature is 500° C., and ...
Embodiment 2
[0035] 1. Light burning. Add magnesite (MgO content is 45.8% by weight) into a roasting furnace, and roast at 750° C. for 90 minutes to obtain light-burned magnesium oxide.
[0036] 2. Cool down. Naturally cool the light-burned magnesia to 250°C in air.
[0037] 3. Hydration. Put the cooled light-burned magnesia into water 4 times the volume of the mineral, mix, add the mixed slurry into the ball mill tank, ball mill for 45 minutes, and then heat the ball-milled slurry to 180°C, Rapid hydration of MgO under temperature conditions to generate Mg(OH) 2 .
[0038] 4. Purification. Purify by filtering, keep the slurry and discard the filter residue.
[0039] 5. Dry. The slurry was dried at 180° C. for 8 hours to remove moisture.
[0040] 6. Ball milling. The dried Mg(OH) 2 Place in a ball mill jar and grind to -325 mesh.
[0041] 7. Calcining at low temperature. Mg(OH) after low temperature calcination and ball milling 2 , the calcination temperature is 500 DEG C, the...
Embodiment 3
[0043] 1. Light burning. Add magnesite (MgO content is 47.0% by weight) into a roasting furnace, and roast at 700° C. for 120 minutes to obtain light-burned magnesium oxide.
[0044] 2. Cool down. Naturally cool the light-burned magnesia to 150°C in air.
[0045] 3. Hydration. Put the cooled light-burned magnesia into water 5 times the volume of the mineral, mix, add the mixed slurry into the ball mill tank, ball mill for 30 minutes, and then heat the ball-milled slurry to 120°C. Rapid hydration of MgO under temperature conditions to generate Mg(OH) 2 .
[0046] 4. Purification. Purify by filtering, keep the slurry and discard the filter residue.
[0047] 5. Dry. The slurry was dried at 120° C. for 15 hours to remove moisture.
[0048] 6. Ball milling. The dried Mg(OH) 2 Place in a ball mill jar and grind to -325 mesh.
[0049] 7. Calcining at low temperature. Mg(OH) after low temperature calcination and ball milling 2 , the calcination temperature is 550° C., and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com