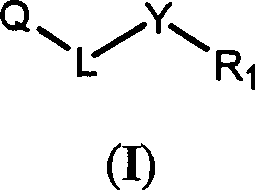
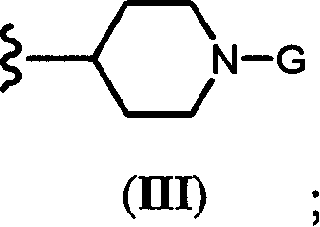
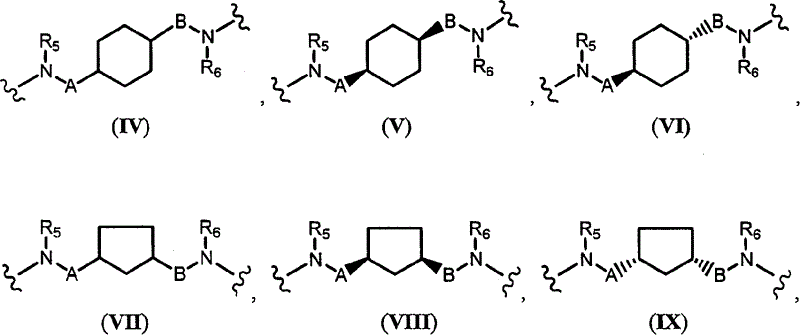
Novel quinazoline derivatives and methods of treatment related to the use thereof
A compound and hydrate technology, applied in the field of MCH receptor antagonist compounds, can solve problems such as energy absorption and consumption imbalance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1669] 1-(3,4-Dimethoxy-phenyl)-3-[cis-4-(4-dimethylamino-quinazolin-2-ylamino)-cyclohexyl]-urea hydrochloride
[1670] Step A: Synthesis of 2,4-dichloro-quinazoline.
[1671] 1H-quinazoline-2,4-dione (150 g, 925 mmol) in POCl 3 (549 mL, 5.89 mol) suspension was added dimethyl-phenyl-amine (123 mL, 962 mmol). The mixture was stirred at reflux for 7 h and then concentrated. The solution was poured into ice water, and the aqueous layer was washed with CHCl 3 Extraction (three times). The combined organic layers were washed with MgSO 4 dried, filtered, concentrated, and purified by flash chromatography (silica gel, 50% CHCl 3 / Hexane to 10% EtOAc / CHCl 3 ) afforded 2,4-dichloro-quinazoline (159 g, 86%) as a pale yellow solid.
[1672] CI MS m / e 199, M + ; 1 H NMR (300MHz, CDCl 3 ), δ7.71-7.81 (m, 1H), 7.95-8.04 (m, 2H), 8.27 (dt, J=8.3, 1.1 Hz, 1H).
[1673] Step B: Synthesis of (2-chloro-quinazolin-4-yl)-dimethyl-amine.
[1674] A solution of 2,4-dichloro-quinazoline ...
Embodiment 2
[1689] 1-(2,3-Dichloro-phenyl)-3-[cis-4-(4-dimethylamino-quinazolin-2-ylamino)-cyclohexylmethyl]-urea hydrochloride
[1690] Step A: Synthesis of (cis-4-hydroxymethyl-cyclohexyl)-carbamic acid tert-butyl ester.
[1691] A suspension of cis-4-amino-cyclohexanecarboxylic acid (244 g, 1.71 mol) in MeOH (2.45 L) was cooled to -8°C. Thionyl chloride (440 mL, 6.03 mol) was added dropwise. The resulting solution was stirred at room temperature for 4.5 h and then concentrated to give a white solid. CHCl of the above solid 3 (3.00L) suspension was added successively triethylamine (261mL, 1.88mol) and (Boc) 2 O (409 g, 1.88 mol). The reaction mixture was stirred at room temperature for 5 h and poured into water. CHCl for aqueous layer 3 Extraction (three times). The combined organic layers were washed with MgSO 4 Dry, filter, concentrate, and flash chromatography (silica gel, 11% EtOAc / hexane to 10% MeOH / CHCl 3 ) and flash chromatography (NH-silica, 33% EtOAc / hexane to 9% MeOH / ...
Embodiment 3
[1709] 1-(2,6-Dichloro-phenyl)-3-[cis-4-(4-dimethylamino-quinazolin-2-ylamino)-cyclohexylmethyl]-urea hydrochloride
[1710] Step A: Synthesis of 1-(2,6-dichloro-phenyl)-3-[cis-4-(4-dimethylamino-quinazolin-2-ylamino)-cyclohexylmethyl]-urea Hydrochloride.
[1711] Following the method of step F of Example 1, the title compound was obtained.
[1712] ESI MS m / e 509, M(free)+Na + ; 1 H NMR (300MHz, CDCl 3 ), δ1.51-2.06(m, 9H), 3.37-3.42(m, 2H), 3.52(s, 6H), 4.37-4.47(m, 1H), 6.35-6.45(m, 1H), 6.96-7.06 (m, 1H), 7.23-7.31(m, 3H), 7.43-7.49(m, 1H), 7.61-7.68(m, 1H), 7.91(d, J=7.9Hz, 2H), 8.72(d, J =8.7Hz, 1H), 12.64(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com