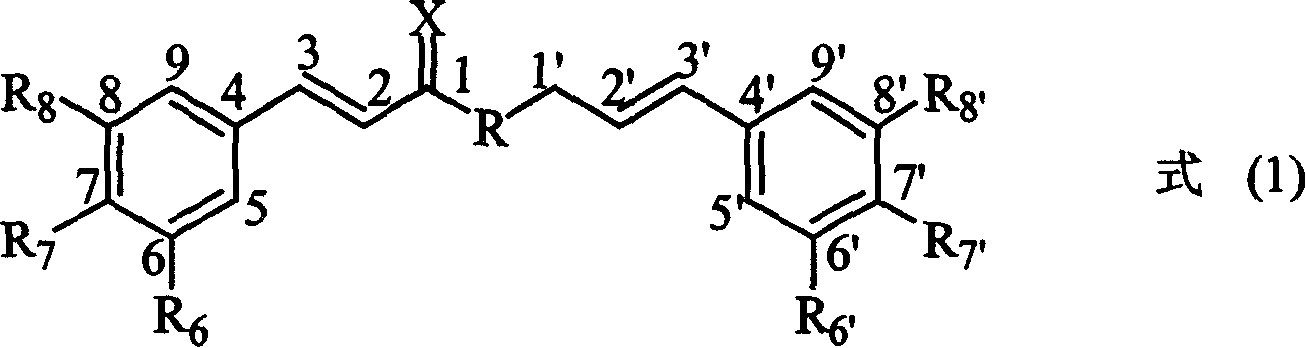
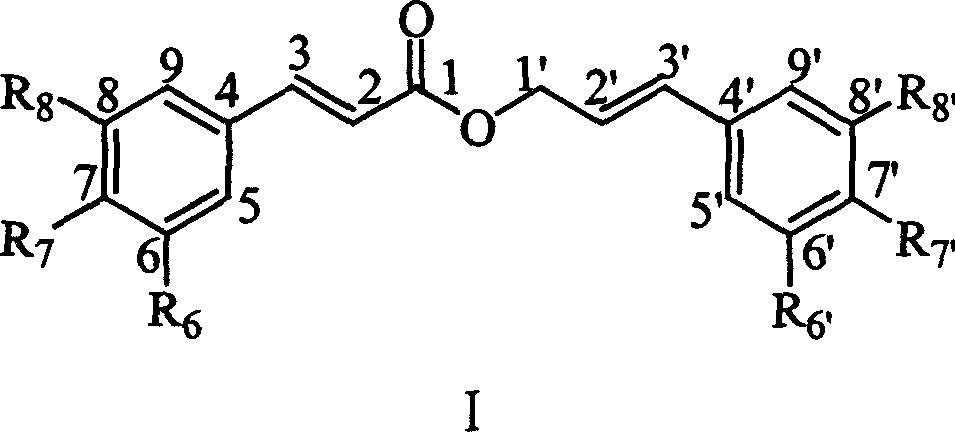
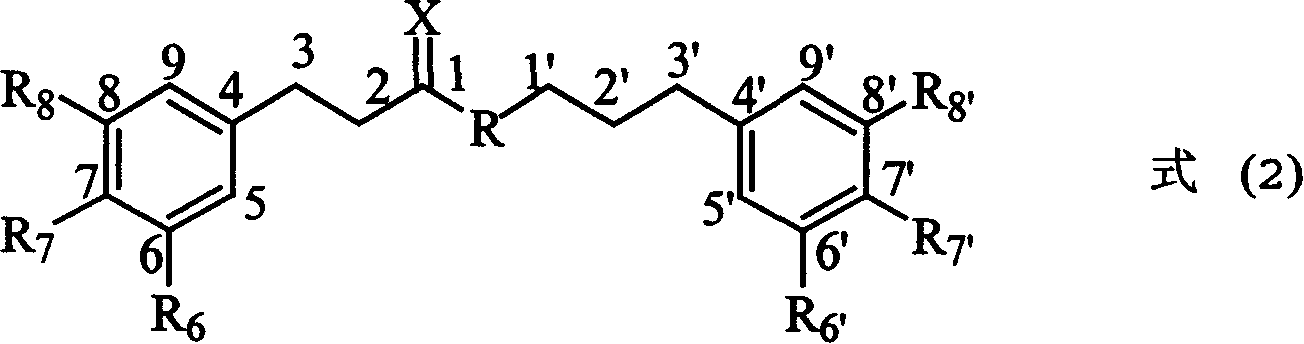
Compounds of class of styracin and cinepazid ester phenylpropionic acid, prepration method and application
A technology of phenylacrylic acid phenylpropylene and phenylacrylic acid, which is applied in drug combination, organic chemistry, drug delivery, etc., can solve the problems of limiting the universal applicability of drugs, low selectivity, and malignant killing of normal cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0092] A further embodiment of the present invention is:
[0093] When the substituent X in the compound of formula (3) is oxygen, the substituent R is ethoxy (CH 3 CH 2 O) during, as shown in formula (4), be formula I-1 compound:
[0094] Formula (4)
[0095] where substituent R 6 , R 7 , R 8 The definition of is the same as formula (3).
[0096] Compounds of formula I-1 of the present invention include:
[0097] I-1-a. (E)-7-methoxyl ethyl phenylpropionate;
[0098] I-1-b. (E)-6,7-Dimethoxymethoxy-ethyl phenylpropionate;
[0099] I-1-c. (E)-6,8-dimethoxy-7-O-(4'-bromobenzyl)-phenylpropionic acid ethyl ester;
[0100] I-1-d. Ethyl (E)-7-nitrophenylpropionate;
[0101] I-1-e. (E) - ethyl 7-methoxymethoxyphenylpropionate;
[0102] I-1-f. (E)-6,8-dimethoxy-7-O-(2'-fluoro, 4'-bromobenzyl)-phenylpropionic acid ethyl ester;
[0103] I-1-g. (E)-6,7-dimethoxy-ethyl phenylpropionate;
[0104] I-1-h. ethyl cinnamate;
[0105] I-1-i. (E)-6,8-dimethoxy-7-O-(3'-bromobenzyl...
Embodiment 1
[0155] Example 1: Preparation of Compound I-i ((E)-(7-methoxymethoxy)phenylpropionic acid-(E)-(7'-methoxymethoxy)phenylpropenyl ester)
[0156]
[0157] This example relates to the general synthesis of compounds of formula (1) with cytotoxic activity. It specifically relates to the synthesis of compound (E)-(7-methoxymethoxy)phenylpropanoic acid-(E)-(7'-methoxymethoxy)phenylpropenyl ester. Dissolve p-methoxymethoxycinnamic acid (292mg, 1.4mmol), N,N-dicyclohexylcarbodiimide (318mg, 1.5mmol), 4-dimethylaminopyridine (34mg, 0.28mmol) in dichloro In methane, it was stirred at room temperature for 10 minutes, and white turbidity appeared, and a dichloromethane solution of p-methoxymethoxycinnamyl alcohol (300 mg, 15 mmol) was added, and stirred at room temperature for 24 hours. Celite was filtered, the filtrate was concentrated, and the crude product was separated by column chromatography (n-hexane / ethyl acetate=8:1, crude product / silica gel=1:50) to obtain 468 mg of a white s...
Embodiment 2-19
[0159] According to the method of Example 1, the compounds of Examples 2-19 shown in the following Table 1 were prepared:
[0160]
[0161] implement
Example number
compound
substituent
R 6
R 7
R 8
R 6′
R 7′
R 8′
2
I-a
H
OCH 3
H
H
OCH 3
H
3
I-b
OCH 3
OCH 3
OCH 3
H
OCH 3
H
4
I-c
H
NO 2
H
OCH 3
OCH 3
H
5
I-d
OCH 3
OCH 3
OCH 3
OCH 3
OCH 3
OCH 3
6
I-e
H
OCH 3
H
OCH 3
OCH 3
H
7
I-f
OCH 3
OCH 3
H
OCH 3
OCH 3
H
[0162]
[0163] List the physicochemical data of each compound in Table 1 below:
[0164] Compound I-a:
[0165] White solid, melting point: 63-64°C, Rf (n-hexane / ethyl acetate: 3 / 1): 0.71; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com