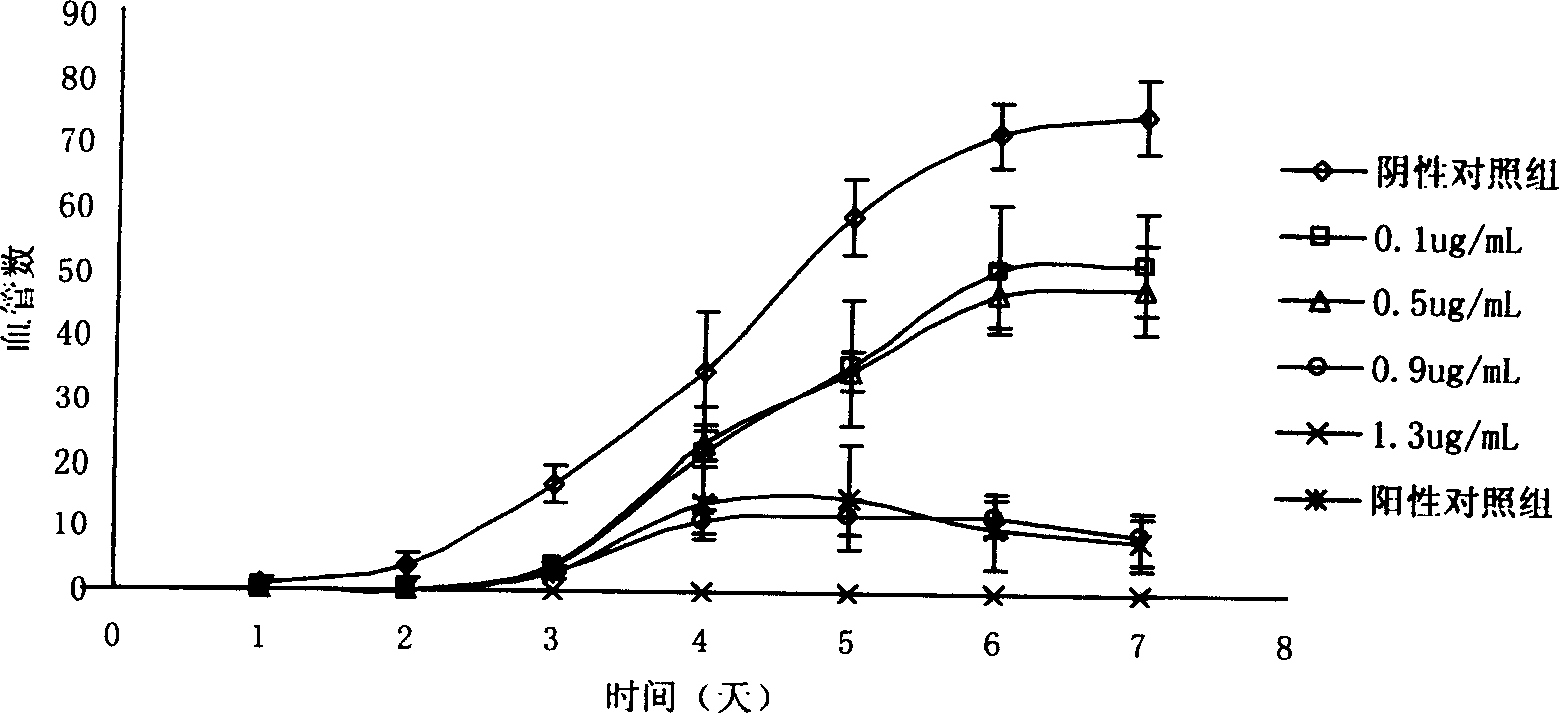
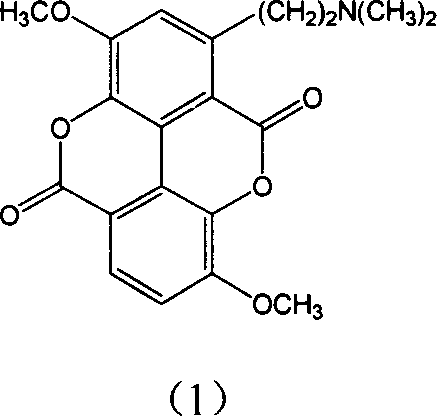
Taspine alkaline preparation method and uses in preparing medicine for treating tumour
A technology of tospinine and alkali solution, which is applied in antineoplastic drugs, drug combinations, active ingredients of heterocyclic compounds, etc., can solve problems such as no application reports of tospinine, and achieve high product purity and simple process steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Taking the used raw material orchids total alkaloids 100g of preparing tospin base as an example, its preparation method steps are as follows:
[0050] 1. Configure the solution
[0051] Prepare 1000 g of hydrochloric acid solution with a concentration of 1% in a conventional method as a solution.
[0052] 2. Dissolving total alkaloids of red hair seven
[0053] Get the total alkaloids 100g of orchids and put them into the reaction kettle, add the concentration of 1% hydrochloric acid solution 1000g, stir with a mixer to make it fully dissolve, and make the total alkaloids of orchids solution.
[0054] 3. Adsorption separation
[0055] Pack the LSD001 type cation exchange resin of 100g on the total alkaloid solution of the red hair seven prepared in step 2 into a column, the column diameter and the column height ratio are 1: 3, the adsorption flow rate is 1.2 times column volume per hour, and the adsorption is carried out at normal temperature and pressure separate. ...
Embodiment 2
[0066] Taking the used raw material orchids total alkaloids 100g of preparing tospin base as an example, its preparation method steps are as follows:
[0067] In this embodiment, in the process step of preparing the dissolving solution, 1000 g of hydrochloric acid solution with a concentration of 2% was prepared as the dissolving solution according to the conventional method. In the process step of dissolving the total alkaloids of orchids, take 100 g of the total alkaloids of orchids and put them into the reactor, add 1000 g of hydrochloric acid solution with a concentration of 2%, stir it with a mixer to fully dissolve it, and make the total alkaloids of orthys anesthesia solution . In the adsorption separation process step, the adsorption flow rate is 1.1 times the column volume per hour, and the adsorption separation is carried out at normal temperature and pressure. Other processing steps are identical with embodiment 1.
Embodiment 3
[0069] Taking the used raw material orchids total alkaloids 100g of preparing tospin base as an example, its preparation method steps are as follows:
[0070] In this embodiment, in the process step of preparing the dissolving solution, 1200 g of hydrochloric acid solution with a concentration of 0.5% was prepared according to the conventional method as the dissolving solution. In the process step of dissolving the total alkaloids of orchids, take 100 g of the total alkaloids of orchids and put them into the reactor, add 1200 g of hydrochloric acid solution with a concentration of 0.5%, stir it with a mixer to fully dissolve it, and make the total alkaloids of orthys anesthesia solution . In the adsorption separation process step, the adsorption flow rate is 1.0 times the column volume per hour, and the adsorption separation is carried out at normal temperature and pressure. Other processing steps are identical with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com